Introducción: El calcio (Ca) es uno de los elementos fundamentales a tener en cuenta en los pacientes en diálisis, dada su relación con el riesgo cardiovascular. Con la introducción de los modernos quelantes del fósforo no cálcicos y de los calcimiméticos, hemos visto variar la calcemia prehemodiálisis, en los últimos años, de 9,5-10,5 a 8,4-9,5 mg/dl. Para valorar de una forma más precisa las variaciones del Ca durante la sesión de hemodiálisis e individualizar su prescripción, el objetivo del estudio fue comparar diferentes concentraciones de Ca en el baño de diálisis, valorando el balance pre y poshemodiálisis y sus implicaciones en el control del metabolismo fosfocálcico. Pacientes y métodos: Se incluyeron 98 pacientes con una edad media de 59,3 ± 15 años, 68 hombres y 30 mujeres. Cada paciente se sometió a dos sesiones de hemodiálisis variando únicamente la concentración de Ca del baño, una sesión con Ca 2,5 mEq/L (grupo Ca25) y otra con Ca 3,0 mEq/L (grupo Ca30). Se determinaron los niveles de Ca, fósforo (P) y paratohormona (PTH) pre y poshemodiálisis, registrando la medicación relacionada. Además se dividieron los pacientes en cuatro subgrupos según los niveles de calcemia prediálisis en Ca < 8,5, 8,5-9,0, 9,0-9,5 y > 9,5 mg/dl para realizar un análisis más individualizado. Resultados: No se observaron diferencias en los valores prediálisis de Ca, 8,81 ± 0,65 (Ca25) y 8,88 ± 0,61 (Ca30); P, 4,01 ± 1,3 (Ca25) y 4,19 ± 1,2 (Ca30); y PTH, 352 ± 288 (Ca25) y 369 ± 310 (Ca30). Con el baño Ca25, el Ca y la PTH posdiálisis no se modificaron significativamente, mientras que con el Ca30 se observó un incremento significativo del Ca a 10,2 ± 0,6 (p < 0,001) acompañado de un descenso de la PTH (181 ± 227, p < 0,001). No obstante, cuando se analizaba el baño Ca25 por subgrupos de Ca prediálisis (< 8,5 mg/dl [30,6%], 8,5-9,0 [31,6%], 9,1-9,5 [23,5%] y > 9,5 mg/dl [14,3%]), se apreció un aumento del Ca posdiálisis en los subgrupos < 8,5 (p < 0,001) y 8,5-9,0 (p < 0,01), sin cambios en el subgrupo 9,1-9,5, y un descenso del Ca posdiálisis cuando los valores iniciales eran > 9,5 mg/dl (p < 0,01). Con el baño Ca30 se apreció un aumento del Ca posdiálisis en todos los subgrupos (p < 0,001) acompañado de un descenso de la PTH posdiálisis (p < 0,01). Un 42% de los enfermos tomaban calcimiméticos, un 47% paricalcitol y un 32% captores de fósforo con Ca, sin relacionar estos fármacos con el comportamiento del Ca pre y posdiálisis estudiados con ambos baños. Conclusión: La prescripción del Ca del baño de diálisis necesita una individualización basada en los valores pre y posdiálisis de Ca y la necesidad de obtener incrementos, descensos o no variaciones en el calcio posdiálisis en relación con la situación clínica del metabolismo fosfocálcico del enfermo.
Calcium is one of the key elements to consider in patients on dialysis due to its relationship with cardiovascular risk. The introduction of non-calcium-based phosphate binders and calcimimetics has changed the setting for pre-dialysis serum calcium in recent years from 9.5-10.5mg/dl to 8.5-9.5mg/dl. To assess more accurately the changes in calcium (Ca) during haemodialysis sessions and to individualise prescriptions, the aim of this study was to assess the intradialytic changes of two different dialysate Ca concentrations before and after hemodialysis and their implications in controlling calcium-phosphate metabolism. Patients and method: We analysed 98 patients with a mean age of 59.3±15 years, 68 of which were men and 30 women. Each patient received two HD sessions with two different dialysate Ca concentrations: 2.5mEq/l (Ca25 group) or 3.0mEq/l (Ca30 group). Pre- and post-dialysis Ca, phosphorus (P) and PTH were determined, and associated medications were recorded. For a more individualised analysis, patients were divided into four subgroups of Ca<8.5mg/dl, 8.5-9.0mg/dl, 9.0-9.5mg/dl, and >9.5mg/dl, according to pre-dialysis serum calcium levels. Results: There were no differences in pre-dialysis values of Ca: 8.81±0.65 (CA25) and 8.88±0.61 (CA30), P: 4.01±1.3 (CA25) and 4.19±1.2 (CA30), or PTH: 352±288 (CA25) and 369±310 (CA30). Post-dialysis Ca and PTH did not change significantly with CA25 dialysate, although there was a significant post-dialysis Ca increase to 10.2±0.6 (P<.001) accompanied by a decrease in post-dialysis PTH (181±227, P<.001) with CA30. However, with CA25 dialysate, when different subgroups of pre-dialysis Ca were analysed: <8.5mg/dl (30.6%), 8.5-9.0mg/dl (31.6%), 9.1-9.5mg/dl (23.5%) and >9.5mg/dl (14.3%) we observed a Ca increase during the session in the <8.5 (P<.001) and 8.5-9.0 (P<.01) subgroups. Ca was unchanged in the 9.1-9.5 group and Ca decreased when the initial Ca values were >9.5mg/dL (P<.01). A Ca increase (P<.001) and a decrease in PTH (P<.01) were observed in all subgroups with CA30 dialysate. A total of 42% of patients were taking calcimimetics, 47% paricalcitol, and 32% calcium-based phosphate binders, although these drugs were not linked with pre- or post-dialysis Ca levels in or dialysate treatment. Conclusion: We concluded that the prescription of Ca dialysate needs to be individualised based on pre- and post-dialysis Ca values and the need for an increase, decrease, or no changes in post-dialysis calcium in relation to the clinical condition of the patient’s phosphorous-calcium metabolism.
INTRODUCTION
Cardiovascular disease is the most common cause of death in patients with chronic kidney disease, with a risk of mortality that is 30 times higher than in the general population, to a great extent due to vascular and soft tissue calcification.1 The appropriate dialysate calcium concentrations for haemodialysis (HD) continues to be a matter of debate; an ideal concentration probably does not exist.2
In the 1960s, when dialysis techniques were first developed, Ca concentrations were arbitrarily set at 2.5mEq/l (1.25mmol/l) since they were the Ca levels observed in human blood. However, doctors observed that patients did not absorb much Ca and ended up with hypocalcaemia. Recognising the relationship between hypocalcaemia and hyperparathyroidism (HPT) at the end of the 1960s, a concentration of 3.5mEq/l was recommended, and this value was maintained for several decades as the standard of choice for controlling HPT.3,4
With the use of calcium-based phosphate (P) binders and the introduction of calcitriol in the treatment of patients on HD, the frequency of hypercalcaemia increased and dialysate Ca concentrations were re-evaluated with the intent of lowering them to 2.5-3.0mEq/l.5 However, the later non-calcium-based phosphate binders and calcimimetics altered the pre-dialysis calcaemia protocol. In this context, the KDOQI guidelines6 recommended target Ca values of 8.4-9.5mg/dl, and the KDIGO guidelines7 later suggested that Ca levels be modified according to the protocols of each laboratory. These objectives were compiled in the Spanish Society of Nephrology’s guidelines for managing mineral and bone disorders in patients with chronic kidney disease.8
In order to evaluate Ca variations more precisely during HD sessions and to individualise dialysate Ca levels, our study compares different dialysate Ca concentrations, pre- and post-haemodialysis changes, and their implications in the control of phosphorous/calcium metabolism.
PATIENTS AND METHOD
Ours was a prospective interventional study with 98 HD patients (68 men and 30 women) with a mean age of 59.3±15 years (range: 24-68 years) who were on chronic treatment for a mean 42.6±49 months. The aetiology of the chronic kidney disease in these patients was: diabetic nephropathy in 17 cases, polycystic kidney disease in 14 cases, ischaemic nephropathy in 21 cases, tubulo-interstitial nephropathy in 5 cases, chronic glomerulonephritis in 16 cases, urological diseases in 4 cases, other causes in 6 cases, and unknown causes in 15 cases.
Patients underwent dialysis treatment with 4008 S or 5008 monitors (Fresenius), 86% using post-dilution on-line haemodiafiltration (OL-HDF) and the other 14% using high-flux HD. Patients were treated with 1.4-1.8m2 helixone or 2.1m2 polyamide dialysers under the following treatment regimens: mean time on dialysis: 317±86 minutes (range: 150-480min); blood flow: 411±32ml/min (350-500ml/min); dialysate flow rate: 612±146ml/min (400-800ml/min); convective transport volume: 23.5±14l (range: 0-55l).
Each patient underwent two dialysis sessions between which the only change was the dialysate Ca concentration: 2.5mEq/l (Ca25 group) or 3.0mEq/l (Ca30 group) in the middle session of each week, with a one-week interval between each session, with no changes in any other HD parameters. The application of 2.5mEq/l or 3.0mEq/l Ca was randomised. For each HD session, we tested patients for Ca, P, and intact parathyroid hormone (PTH) levels before dialysis (directly withdrawn from the patient) and post-dialysis (withdrawn from the arterial blood outflow after waiting one minute with a 50ml/min blood flow rate). PTH was analysed using DiaSorin® reactants. The coefficient of variation in Ca measurements was 1.64%, and for P, 1.98%. We did not adjust post-dialysis calcaemia values for blood concentration or changes in albumin concentrations.
We also recorded the medications taken by each patient associated with Ca-P metabolism (calcimimetics, paricalcitol, and phosphate binders).
Results were expressed as arithmetic mean ± standard deviation. For the statistical analysis of quantitative variables, we used Student’s paired or non-paired t-tests, or ANOVA tests for repeated measures (Newman-Keuls test). We considered a P-value of <.05 as statistically significant.
RESULTS
We examined a total of 196 sessions, all of which were well tolerated, and observed no episodes of hypotension, hypertension, or arrhythmia attributable to changes in dialysate Ca concentrations.
Mean pre-dialysis Ca was 8.81±0.65mg/dl for the Ca25 group and 8.88±0.61mg/dl for the Ca30 group. Mean pre-dialysis P was 4.01±1.3mg/dl and 4.19±1.2mg/dl for the Ca25 and Ca30 groups, respectively. Mean pre-dialysis PTH was 352±288pg/ml and 369±310pg/ml, respectively.
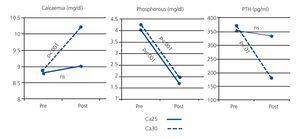
As shown in Figure 1, pre- and post-dialysis Ca values in the Ca25 group did not differ significantly, whereas we did observe a significant increase in the Ca30 group. Post-dialysis P was significantly reduced in both groups.
Variations in Ca during dialysis sessions were accompanied by inverse changes in PTH. For the Ca25 group, post-dialysis PTH did not vary significantly: 352±287pg/ml pre-dialysis versus 332±289pg/ml post-dialysis. In the Ca30 group, the mean pre-dialysis PTH value of 369±311pg/ml was reduced to 181±227pg/ml after dialysis (P<.001), due to the inhibition caused by the higher post-dialysis calcaemia in this treatment group (Fig. 1).
Analysis by sub-groups: individualised calcium balance
With the goal of achieving a more individualised characterisation of patient results, we divided patients according to pre-dialysis Ca values and performed a subsequent analysis of the following sub-groups: Ca<8.5mg/dl (sub-group 1), 8.59.5mg/dl (sub-group 4).
Ca25 group
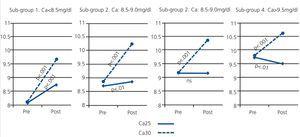
Sub-group 1 (Ca<8.5mg/dl), n=30. Starting with a mean Ca of 8.07±0.44, post-dialysis calcaemia increased to 8.74±0.41mg/dl, P<.001 (Fig. 2).
Of the 30 patients in this sub-group, post-dialysis Ca values increased in 25 (83.3%) patients, remained stable in 2 (6.7%), and decreased in 3 (10%) patients.
Sub-group 2 (Ca: 8.5-9.0mg/dl), n=31. The mean pre-dialysis Ca of 8.75±0.17 increased to 9.01±0.48mg/dl after dialysis; P<.01 (Fig. 2).
Of the 31 patients in this sub-group, post-dialysis Ca values increased in 19 (61.3%) cases, remained stable in 2 (6.4%), and decreased in 10 (32.3%) patients.
Sub-group 3 (Ca: 9.0-9.5mg/dl), n=23. We did not observe significant differences between pre- and post-dialysis values (Fig. 2).
Of the 31 patients in this sub-group, post-dialysis Ca values increased in 9 (39.1%) cases, remained stable in 3 (13%), and decreased in 11 (47.8%) patients.
Sub-group 4 (Ca>9.5mg/dl), n=14. The pre-dialysis Ca value of 9.77±0.22 decreased slightly to 9.33±0.41mg/dl after dialysis, P<.01 (Fig. 2).
In this sub-group, post-dialysis Ca values increased in only 1 patient (7.1%), showed no change in another one (7.1%), and decreased in all others (85.7%).
P values decreased in all sub-groups at a similar rate, regardless of pre-dialysis Ca levels.
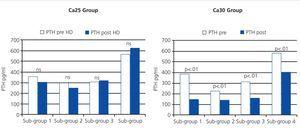
Pre- and post-dialysis PTH values in the four different sub-groups were not significantly different (Fig. 3).
In sub-group 4, patients with extremely high pre-dialysis calcaemia also had higher P and PTH levels, showing that this is the most difficult group to manage in terms of phosphorous-calcium metabolism.
Ca30 group
When the dialysate Ca levels were 3.0mEq/l, all sub-groups experienced a significant increase in post-dialysis Ca (Fig. 2). These differences were milder in sub-groups that started with a higher pre-dialysis Ca value: increases of 19.2%, 16.3%, 13.0%, and 7.5% in sub-groups 1, 2, 3, and 4, respectively.
In sub-group 4, this increase in post-dialysis Ca was accompanied by a significant reduction in post-dialysis PTH values (Fig. 3).
P levels decreased in all sub-groups in a similar manner, as was the case in the Ca25 treatment.
Influence of medication
With the objective of evaluating if medications for the control of phosphorous/calcium metabolism influenced the behaviour of Ca during HD sessions, we analysed whether or not the patients received paricalcitol (100% intravenous), cinacalcet, or Ca-based phosphate binders.
The Table shows pre- and post-dialysis Ca, P, and PTH values in patients receiving paricalcitol, cinacalcet, or calcium-bases phosphate binders. This medication only had a very slight influence on calcaemia variations during dialysis sessions. The main determining factor for changes in calcaemia was the dialysate Ca concentration.
Influence of duration of dialysis
As regards the duration of dialysis, 76% of patients were dialysed for 4-5 hours, 19% for 8 hours (long nocturnal dialysis), and 5% for 3 hours (daily dialysis).
Patients that were dialysed for less than 3 hours had similar post-dialysis Ca values to those undergoing 4-5 hours or more than 8 hours of dialysis, with post-dialysis Ca values of 9.26±0.4mg/dl vs 8.94±0.6mg/dl and 9.13±0.5mg/dl (non-significant [ns]) when patients were treated using the Ca25 protocol. However, when the dialysate concentration was Ca30, post-dialysis Ca increased significantly regardless of the duration of each dialysis session (Table). As such, we observed that the duration of dialysis had little influence on changes in calcaemia during the dialysis session and that intradialytic changes in Ca depended on which dialysate Ca concentration was used and, to a lesser degree, pre-dialysis Ca levels.
Influence of dialysis technique
As regards the type of dialysis treatment, 14% of patients received high-flux HD and 86% received OL-HDF, with a reinfusion volume of 24.1±14 litres.
The Table displays Ca, P, and PTH levels both pre- and post-dialysis, arranged by type of dialysis. Here we also observed that the dialysis technique had little influence on calcaemia variations during dialysis sessions, reinforcing the idea that the primary determining factor was the dialysate Ca concentration.
DISCUSSION
Homeostasis of Ca and P metabolism and secondary HPT are very important for the control of renal failure and dialysis patients in general. Increased serum P concentrations and Ca x P product is recognised as a mortality factor due to the increased cardiovascular risk in this population.9
Choosing an appropriate Ca concentration for dialysate is critical for managing patients on dialysis. A positive Ca balance is associated with vascular calcifications, while Ca loss can trigger HPT and decreased bone mass.10
Currently, three different Ca concentrations are commercially available for dialysate in HD: Ca 1.25mmol/l (2.5mEq/l), Ca 1.5mmol/l (3.0mEq/l), and Ca 1.75mmol/l (3.5mEq/l). No consensus exists for what the ideal dialysate Ca concentration is; there probably is no single ideal concentration.2 Low Ca concentrations (1.25-1.50mmol/l) have the advantage of reducing the risk of hypercalcaemia, allow for the use of vitamin D and calcium-based phosphate binders, and prevent adynamic bone disease.11 The disadvantages include poor haemodynamic tolerance,11 greater risk of arrythmia,11,12 and stimulation of PTH with the development of secondary de novo HPT or exacerbations in patients that already have HPT.13,14 High Ca concentrations offer better control of secondary HPT but also bring the risk of hypercalcaemia if the patient is not strictly monitored.2,11
Ca concentrations of 2.5mEq/l were first used because of the similarity to serum concentrations, but it was observed that patients absorbed little Ca and frequently developed hypocalcaemia. Recognising the relationship between hypocalcaemia and HPT, the use of concentrations of 3.5mEq/l was promoted towards the end of the 1970s, and this practice was maintained for a long period of time as the standard protocol for controlling HPT and reducing the frequency of hypocalcaemia.15
With the arrival of calcitriol, which improved intestinal intake of Ca, and the gradual introduction of calcium-based phosphate binders replacing aluminium-based ones, excessive Ca uptake was observed, with the associated risks of the previously mentioned complications. This, together with an increased prevalence of adynamic bone disease, which was associated with positive Ca balances, led to the recommendation in recent decades for using a dialysate Ca concentration of 2.5mEq/l.16
The recommendations from clinical guidelines for managing phosphorous/calcium metabolism have evolved in recent years in light of new findings and studies. The KDOQI guidelines recommended Ca 2.5mEq/l in 2003; two years later, the same guidelines recommended using Ca 2.5mEq/l dialysate in patients treated with Ca-based chelating agents and Ca 3.0mEq/l for patients not receiving calcium salts, taking into account serum Ca levels and the use of vitamin D analogues.6 The KDIGO guidelines in 2009 left this decision to the criteria of the attending physician, since no exact criteria are given for one concentration of Ca or another.7
In this study, we analysed patients based on pre-dialysis calcaemia levels, as the starting point for individualised treatment, and evaluated how the use of a dialysate concentration of Ca25 or Ca30 influenced post-dialysis Ca and PTH values. In the Ca25 group, we observed that patients with lower pre-dialysis calcaemia had a slight increase in post-dialysis Ca, with a tendency for reduced PTH; pre-dialysis patients with Ca values of 8.5-9.5mg/dl did not experience changes between pre- and post-dialysis Ca and PTH values, and patients with baseline Ca values >9.5mg/dl showed a slight decrease in post-dialysis Ca, with a tendency for increased PTH. However, in the Ca30 group, we observed a post-dialysis increase in Ca and an important reduction in PTH, and both of these results were more notable in patients with lower pre-dialysis Ca values. These observations of the behaviour of post-dialysis Ca and its close relationship with post-dialysis changes in PTH suggest that measuring post-dialysis Ca could be an easy, practical, and accessible tool for evaluating intradialytic variations in Ca and PTH, which would allow for an individualised evaluation of the repercussions of dialysis prescription, specifically, the dialysate Ca concentrations.
However, we must be cautious when interpreting the post-dialysis results for Ca and PTH, and we also must keep in mind the kinetics of Ca metabolism.17 During dialysis, various changes are produced that affect post-dialysis Ca, such as variations in Ca bound to proteins that affect pH, bicarbonate, and other ions, haemoconcentration, and finally, the rebound process or post-dialysis inter-compartmental balance, which can take up to 180 minutes following dialysis sessions. In this sense, several studies observed an increase in mid- to long-term HPT when Ca 2.5mEq/l is used as a standard value. Al Aly et al18 and Fernandez et al19 demonstrated how patients receiving dialysis with Ca25 dialysate developed higher PTH values in the long-term, with an increase of up to 75% of cases of HPT. Molina et al20 compared mid-term results of switching dialysate Ca from a 2.5mEq/l to 3.0mEq/l and observed that, in the absence of hypercalcaemia or excessive PTH suppression, patients treated with 3.0mEq/l dialysate Ca concentrations had a better control of HPT, without risk of severe hypercalcaemia.
The OL HDF convective techniques use the same dialysate as a replacement fluid, and as it contains Ca, has an impact on Ca control. In this study, we observed that, when using a Ca25 concentration, there were no changes in final Ca between patients undergoing HD or OL HDF. When using a Ca30 concentration, the increase in final Ca was slightly higher in patients on OL HDF than in those on HD (15.1% vs 13.2%, respectively) with a similar reduction in PTH (50.5% vs 53.0%). As such, the type of dialysis has little or no influence on intradialytic variations in Ca. Malberti et al21 reinforced these results by demonstrating little difference between patients treated with HD and OL HDF, despite the slightly negative ionic Ca balance observed in patients on both types of dialysis.
The dynamics of Ca-P-PTH balance is always changing, and choosing one Ca concentration or another for dialysate should be a decision made based on the patient’s tendencies and the progression of laboratory results. It is not advisable to make decisions based on isolated laboratory results without evaluating these in the context of the patient. In this sense, we should point out that phosphorous/calcium control has improved in recent years thanks to the development of paricalcitol- and cinacalcet-based treatments.22 The effect of these drugs mainly affects the inter-dialysis period; in our study, we observed very little influence of these drugs on intradialytic changes in terms of calcaemia and PTH.
To conclude, the individualisation of dialysate Ca concentrations should be recommended in order to adequately control calcification in patients on dialysis, which depends on the clinical, dynamic, and individual situation of chronic kidney disease patients. Evaluating pre-dialysis and post-dialysis calcaemia can be of great help in the individualisation of dialysate concentrations.
Conflicts of interest
The authors affirm that they have no conflicts of interest related to the content of this article.
Table 1. Influence of pharmacological treatment, duration of dialysis, and mode of dialysis on phosphorous/calcium metabolism in pre- and post-dialysis periods
Figure 1. Comparison of calcaemia, phosphataemia, and intact parathyroid hormone in pre- and post-dialysis periods in patient groups receiving dialysate at Ca25 and Ca30 concentrations
Figure 2. Comparison of pre- and post-dialysis calcaemia in groups receiving dialysate at Ca25 and Ca30 concentrations in the different subgroups of pre-dialysis calcium levels
Figure 3. Comparison of pre- and post-dialysis values of parathyroid hormone analysed in groups receiving dialysate at Ca25 and Ca30 concentrations in the different sub-groups of pre-dialysis calcium levels