Introducción: el objetivo de este trabajo es comunicar nuestra experiencia en el rescate o salvación de los accesos vasculares para hemodiálisis trombosados (fístulas autólogas e injertos protésicos) mediante técnicas de radiología vascular intervencionista. Material y métodos: en los últimos cuatro años hemos tratado radiológicamente 101 accesos vasculares para hemodiálisis trombosados, 44 fístulas autólogas (43,56%) y 57 injertos protésicos (56,44%). La distribución por sexos fue de 69 hombres (68,3%) y 32 mujeres (31,7%), con una edad media de 67,63 años (r: 33-84). La antigüedad media del acceso desde su realización quirúrgica fue de 23,79 meses (r: 1-132). La técnica de rescate fue la tromboaspiración manual con catéter con presión negativa. En ningún caso se han fragmentado, triturado o empujado los trombos hacia la circulación. Resultados: en total, se rescataron con éxito 78 accesos (77,2%). El porcentaje de éxito en las fístulas nativas fue del 84,44%, y el de injertos protésicos, del 71,42%. En todos los accesos, menos en seis (5,9%), se hizo angioplastia en una o en más lesiones tras la trombectomía. En 14 accesos (13,9%), se implantaron una o más endoprótesis metálicas (stent). El seguimiento medio fue de nueve meses (rango: 0-44). La permeabilidad primaria global fue de 42,3% ± 5 a los seis meses, y de 32% ± 4 al año. Por grupos, en las fístulas nativas las permeabilidades primarias fueron mejores que en los injertos protésicos (p <0,05). Conclusiones: en nuestra opinión, y basándonos en nuestra experiencia, los resultados de rescate de accesos vasculares trombosados son mejores en las fístulas autólogas que en los injertos protésicos. Los buenos resultados obtenidos justifican el rescate mediante técnicas de radiología intervencionista, independientemente del tiempo transcurrido de la trombosis.
Background: The purpose of this paper is to communicate our experience in the salvage of thrombosed haemodialysis vascular accesses using interventional radiology techniques. Methods: In the last four years, we have treated, by radiological means, 101 thrombosed haemodialysis vascular accesses. There were 44 autologous arteriovenous fistulas (43.56%) and 57 PTFE grafts (56.44%). There were 69 men (68.3%) and 32 women (31.7%). The mean age was 67.73 years (range 33-84). The mean vascular access age was 23.79 months (range 1-132). Manual catheter-directed aspiration was used. Fragmented, triturated or pushed the thrombus against the pulmonary circulation was avoided in all cases. Results: 78 accesses were salvaged (77.2%). Autologous fistulas average and PTFE grafts success rate were 84.44% and 71.42% respectively. Angioplasty in one or more lesions after thromboaspiration was performed in all accesses, except six (5.9%). Metallic endoprostheses were implanted in 14 accesses (13.9%). Mean follow-up was 9 months (range 0-44). Primary patency was 42.3% ± 5 at 6 months and 32% ± 4 at one year. Autologous fistulas patency was better than PTFE grafts patency (p ≤0,05). Conclusions: Our results suggest thrombosed autologous arteriovenous fistulas salvage is better than PTFE grafts. This justifies interventional radiology techniques in these situations.
INTRODUCTION
Thrombosis in vascular accesses for haemodialysis is a complication that can result in losing the accesses. In more than 85% of all thrombosis episodes, the cause is local stenosis formation, particularly in the most proximal segment to the arteriovenous anastomosis for autologous fistulas, and in venous anastomosis, in the case of PTFE grafts.1-3
Stenosis of access arteries may also cause thrombosis.4 In a small percentage of all cases, thrombosis is caused by hypotension, extrinsic pressure, trauma, infections, or conditions of hypercoagulability.5,6 Clinically, some findings may suggest a stenotic access, such as the presence of dilated segments along the vein, oedema of the extremities or the presence of a pulsatile mass without thrill. Other indicators were detected during haemodialysis sessions, such as inefficiency during dialysis, blood flow deficit, increase in venous pressure, prolonged time to haemostasis after removing dialysis needles, and signs of recirculation. When these events are diagnosed, an intervention is justified with the aim of maintaining the permeability of the access, thus reducing the haemodialysis failure rate due to loss of the access.7,8
Rescue of thrombosed vascular accesses may be achieved by surgical methods or by interventional vascular radiology. The advantage of radiology is that it is a less invasive method and does not consume the patient’s venous reserve.8 There are several radiological techniques for clearing thrombosed vascular accesses. In this article, we describe our experiences gained over the last four years with the Manual catheter-directed aspiration technique, either with or without dilation with an angioplasty balloon and implantation of a stent.
MATERIAL AND METHODS
The Vascular Interventional Radiology Unit at Reina Sofía Hospital in Murcia is the unit of reference for vascular access for haemodialysis in our region, except for one area. It includes three leading hospitals and their corresponding Haemodialysis Units, and seven peripheral dialysis centres.9 Since October 2003, we have been using the manual catheter-directed aspiration technique for thrombosed vascular accesses. Up until then, we had been using different methods to clear accesses, including the use of thrombolytic drugs.
Population
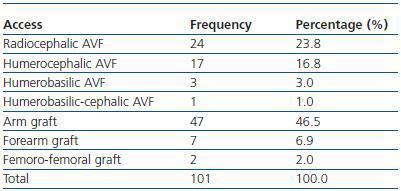
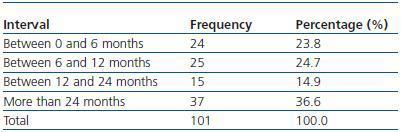
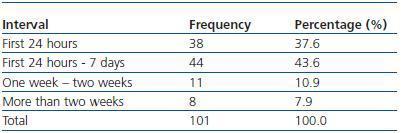
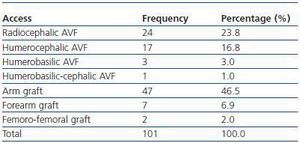
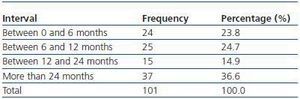
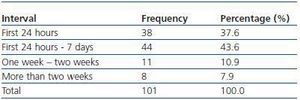
We have used manual catheter-directed aspiration to treat 101 thrombosis cases in 91 thrombosed vascular accesses, 45 autologous fistulas (44.55%) and 56 prosthetic grafts (55.44%) (table 1). Distribution by sex was 69 men (68.3%) and 32 women (31.7%) with a mean age of 67.63 years (r:33-84). The mean duration of use of the access since it was created surgically was 23.79 months (r: 1132) (table 2). Of the 101 episodes of thrombosis, two PTFE grafts presented three thrombotic episodes and eight accesses (six grafts, two AVFs) suffered two episodes of thrombosis. The other 81 accesses only suffered from one episode of thrombosis. Elapsed time from the thrombotic episode to the thrombectomy ranged between 0 and 34 days (mean: 4.96 days) (table 3). Most of the accesses (66.4%) were located in the upper left extremity. The most frequent cardiovascular risk factor was arterial hypertension (52.5%). Most of the accesses (66.4%) were located in the upper left extremity.
Patient preparation
Thrombosed vascular accesses are handled according to the protocol determined by the consensus of the Nephrology Department and our Unit, which is as follows: whenever possible, we attempt to rescue the access on the day of the thrombosis. When this is not possible, the Nephrology Department implants a temporary femoral catheter and we wait until the Vascular Radiology Unit can attend to the patient. The waiting time is no obstacle for attempting to rescue the access.
Once the patient is referred to our Unit, we perform a physical examination of the access and the extremity in which it is located to rule out the existence of inflammatory conditions, which are a categorical counter-indication for thrombectomy. We also study the entire access with ultrasound to evaluate the arterial and venous networks, the presence of aneurisms, the extension of the thrombosis and whether or not collaterals exist. Once the treatment has been planned, the patient is prepared in a completely sterile way, as for a surgical procedure. Before commencing to puncture the access, we must have a coagulation study and the most recent haemogram available. An entryway is chosen on the patient (a vein on the back of the opposite hand) or the catheter is used if applicable, using maximum asepsis. When the procedure is finished, if the patient required immediate dialysis, he or she is moved with the two catheter introducers, which are used in the patient’s dialysis. If dialysis is not needed the same day, the catheter introducers are removed and haemostasis is achieved with a tourniquet system, which is to be removed the following day by the centre’s nursing staff. We recommend lowmolecular- weight heparin on non-dialysis days, and if there is a high risk of thrombosis or the patient has suffered more than one unexplained thrombotic episode, oral anticoagulants as well. All patients are given appointments for ultrasound check-ups.
Manual catheter-directed aspiration
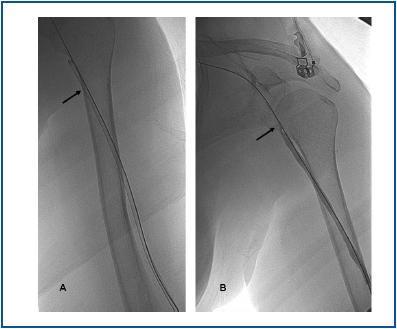
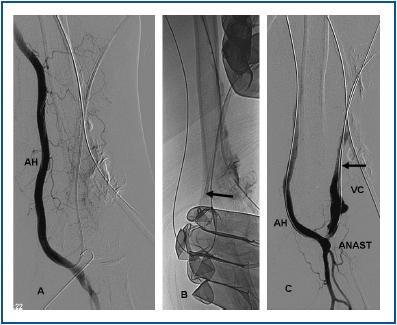
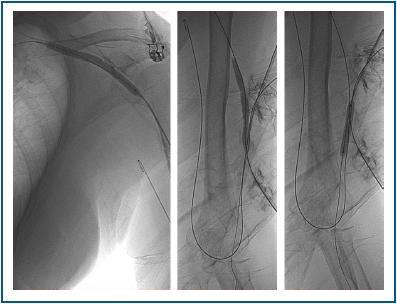
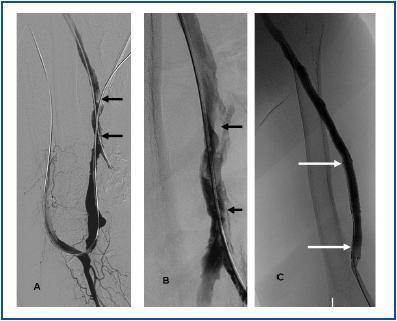
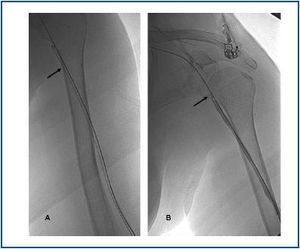
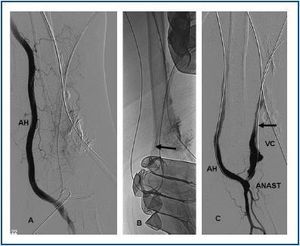
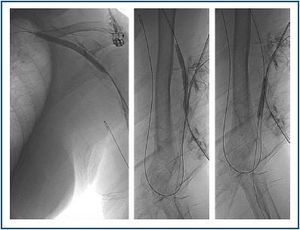
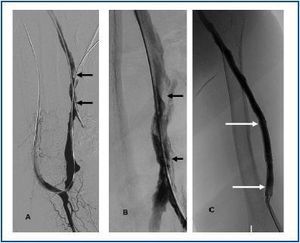
First, using ultrasound monitorisation, the access is threaded in the opposite direction from the arterial anastomosis. A guide wire and catheter is used to clear the obstructed segment to connect with the venous area free from thrombus. For PTFE grafts, this area is generally distal to the venous anastomosis. A safety wire is placed and we begin to aspirate thrombi with a large 7 to 9 French catheter (figures 1 and 2). Once the segment is free from thrombi, the catheter is threaded toward the arterial anastomosis and the same operation is repeated. When the access is free from thrombi, we inflate the underlying stenotic areas responsible for the thrombosis with an angioplasty balloon (figures 3 and 4). Medication during the procedure consists of midazolam as a sedative, an antibiotic (third-generation cephalosporin) and a heparin bolus with 3000-5000 IU of sodium heparin. If the patient needs dialysis immediately, the catheter introducers are left in and dialysis is performed using them. LMWH is recommended on non-dialysis days.
Definitions
In agreement with the standards of the Society of Interventional Radiology,10 we have established the following definitions: an arteriovenous access is autologous if a connection between an artery and a vein is made. An arteriovenous access is not autologous if the union between an artery and a vein is made by placing a tube made of PTFE, Dacron, or biological material (bovine vein or human umbilical cord). Anatomical success of the thrombosed access that has undergone manual thrombus aspiration consists of re-establishing blood flow and obtaining residual stenosis of less than 30% after angioplasty. Haemodynamic success is when the access flow rate increases to the standard recommended levels. Clinical success after thrombus aspiration is when at least one dialysis session is achieved and the clinical examination finds thrill (not a pulsating mass) in the entire access from the arterial anastomosis. Post-intervention primary permeability is the interval between the rescue procedure for the thrombosed access until there is a new thrombus or intervention. Secondary permeability spans the interval between the rescue procedure for the thrombosed access and loss of the same due to it being impossible to maintain, whether this is due to kidney transplant, surgery or loss in the follow-up.
Statistics
The statistical program we used was SPSS for Windows, version 11.0. Standard deviation was used to find the means.
The Kaplan-Meier method was used to calculate permeabilities. The Student T test was used to determine statistical significance, and the Log Rank test for correlations.
RESULTS
Of the 101 thrombosed vascular accesses, 78 were rescued, for a clinical success rate of 77.2%. For autologous fistulae, the rescue rate was 38 of 45 (clinical success of 84.44%) and for prosthetic grafts the rescue rate was 40 of 56 (clinical success of 71.42%). There were no differences in the rescue success rate relating to the elapsed time between the thrombosis of the access and it being rescued using manual catheter-directed aspiration (not statistically significant). As the learning curve for the manual catheter-directed aspiration technique progressed, our results improved. Therefore, for last year, the percentage saved was 96.52% (figure 1).
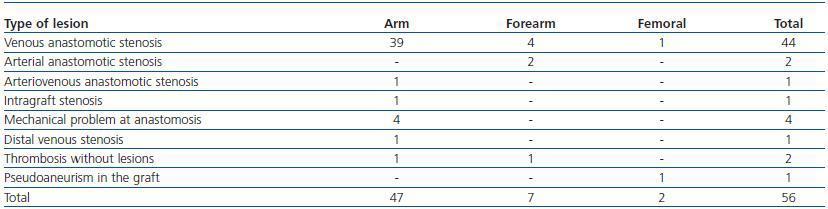
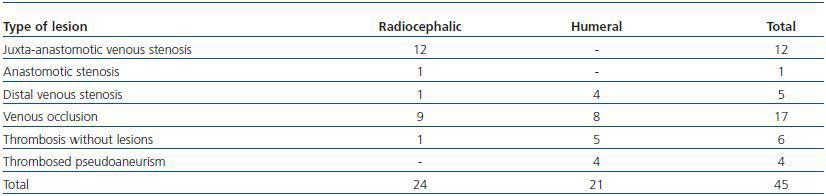
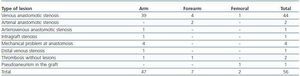
Following thrombus extraction, the most commonly occurring residual culprit lesion for thrombosis has been post-anastomotic venous stenosis for radiocephalic fistulae and stenosis of the venous anastomosis for PTFE grafts. In six cases of autologous fistulas (13.63%) and in two cases with PTFE grafts (3.57%) there was no underlying lesion (tables 4 and 5).
In 81 cases (80.2%) the thrombectomy was accompanied by angioplasty of the underlying lesion or lesions; in 14 cases (13.9%), in addition to thrombectomy and angioplasty, one or more stents was implanted; and in six cases (5.9%) thrombectomy was performed alone.
We have not had any documented pulmonary thromboembolism. In two cases (6%) thrombi entered the arterial circulation, but were successfully aspirated. In one case (3%), a haematoma developed in the puncture area of the humeral artery, and resolved spontaneously. In four cases (12%) a transfusion was needed due to a drop in haematocrit.
Follow-up time on accesses ranges between 0 and 56 months (mean 13 months). Respective primary and secondary permeabilities at 12 months in radial and humeral fistulae were 56.5% ± 10 and 65% ± 10 in the radials, and 37.5% ± 12 and 60.9% ± 12 in the humerals (p ≤ 0.05) (figures 1 and 2). For PTFE grafts, the primary and secondary permeabilites at 12 months were 18.15% ± 5 and 37.4% ± 14 (p ≤ 0.05). Primary permeabilities did not vary according to the elapsed time between the thrombosis and the rescue procedure (42% ± 7 per year, in the group of those who underwent the rescue procedure in the week following thrombosis, and 41% ± 8 per year, in the group of those who underwent the rescue procedure more than a week after the event) (p = 0.873).
At the end of follow-up on the successfully rescued accesses, 48% of the autologous fistulae and 35% of the PTFE grafts were problem-free during dialysis.
DISCUSSION
Vascular access thrombosis for haemodialysis is the complication feared the most by those who work in access care and maintenance. The thrombosis renders the access unusable and obliges us to catheterise if the patient is in urgent need of dialysis. The best outlook is for the thrombosed access to be rescued and salvaged as urgently as possible so as to be able to use it for dialysis. However, this is not always possible. In our experience, we have performed rescue procedures several weeks after the access became thrombosed, without the elapsed time affecting either the immediate success of the procedure (table 3) or the permeabilities. Therefore, the time that passes between the thrombotic episode and the thrombectomy is not a determining factor when it comes to rescuing a thrombosed access. Therefore, the main concern, in our opinion, is saving the access, regardless of the time that has passed since its thrombosis.
The manual catheter-directed aspiration technique was fully and successfully developed by Turmel-Rodrigues in France several years ago.11 In Spain, as far as we know, we were the first to use this technique with vascular access.12 It is not a very costly technique compared with other techniques for removing obstructions. However, it is laborious and requires effort and dedication, and has a learning curve. The evolution of our results, which continue progressing toward a success rate of 100%, confirm this opinion.13
Due to the systematic nature of the technique, which begins with aspirating thrombi distal to the arterial anastomosis, the probability of pulmonary thromboembolism is practically nil. In the experience of the authors who have published their studies to date,14 deliberate manoeuvres that fragment thrombotic material and push it toward the pulmonary circulation are totally unjustified, since deaths by pulmonary thromboembolism have been described.15,16
On the other hand, recurrent pulmonary thromboembolisms in these patients -even subclinical ones- can lead to venous hypertension. One of the principal advantages of the manual catheter-directed aspiration technique, compared to other clearing techniques (particularly those that use pre-designed catheters) is that it is more feasible for use in autologous fistulae. Unlike PTFE grafts, which are rigid tubes, autologous fistulae are veins whose endothelium can be damaged by threading less flexible devices through them.17 Furthermore, these veins can be twisting, aneurismatic and with collaterals, which makes it difficult to thread through existing thrombectomy devices. With the manual catheterdirected aspiration technique, placing a safety wire traces and opens the way more easily. The complementary assistance of an ultrasound with fluoroscopy improves internal and external visibility of the access during the procedure. From the above, we can consider that our results were good, both for autologous fistulas (84.44% success rate) and for PTFE grafts (71.42% success rate).
We have not used pharmacological thrombolysis methods, whether urokinase or rt-PA, although published series show their therapeutic effectiveness.18 We feel that in most cases, above all in autologous fistulae, the fibrinolytic agent is incapable of smoothing all of the thrombotic material, and the occasional appearance of bleeding during perfusion forces the treatment to be suspended.
The use of stents is barely documented for cases of thrombosed dialysis arteriovenous fistulas.19-21 We have implanted them in aneurismatic dilations where manual catheter-directed aspiration was unable to extract all of the material, owing to the presence of thrombi adhering to the venous wall. These thrombi are thrombogenic in themselves, apart from the risk of coming lose and causing a pulmonary embolism. Therefore, in these cases, stents are useful as they compress them against the venous wall, and so rectify aneurisms, reduce their size and decrease risk of embolism. These stents or metal endoprostheses may or may not be coated with synthetic material, such as PTFE or Dacron. Although they are present in dialysis needle puncture zones, they are not an obstacle for them. When possible, we recommend not introducing a needle above the stent during at least two weeks.22 However, if there is not much free tract left in the skin, needles can be inserted from the moment the stent is implanted. The nursing staff’s sensation when puncturing a stent is similar to that felt when puncturing a synthetic PTFE graft. Stents may create hyperplasias at their endpoints or interior and act like stenosis; if this is detected in check-ups, they can be dilated with an angioplasty balloon to improve access permeability.
It is important to run haematological checks on patients who undergo the manual catheter-directed aspiration technique, since frequently blood is extracted along with the thrombi.
This could cause haematocrit to drop two or three points, making a transfusion necessary.
Regarding pharmacological means of preventing another thrombosis episode, we recommend low molecular weight heparin on non-dialysis days. The use of anti-platelet agents is promoted by several studies,23 but we cannot say it is useful based on our own experience. In addition, for those cases in which we suspect an early thrombosis relapse, or in others in which relapse is frequent, we do use dicumarinic anticoagulants.
One high priority is follow-up.24 All of the thrombosed accesses that were successfully rescued receive periodic check-ups in our Interventional Radiology Unit and are studied by ultrasound with a flow meter. In cases in which we suspect stenosis relapse, we confirm it in the angiography room with a fistulography and a new flow measurement for the access taken with an endovascular catheter.25 If stenosis relapse and decreased access flow are confirmed, we perform an angioplasty with balloon on the lesions. The result of the above is that our thrombosis relapse rate26 is 0.05 thrombotic episodes per year for autologous fistulae (0.25 per year is recommended by the SEN (Spanish Society of Nephrology) and by DOQI) and 0.35 per year for PTFE grafts (0.5 per year is recommended by SEN and DOQI).8,27
In conclusion, in presence of experienced vascular radiologists the rescue of thrombosed vascular accesses, whether autologous fistulae or prosthetic grafts, is a possibility of extending the useful life of those accesses. Our results show that all thrombosed vascular accesses may be rescued. Although it is widely accepted that access rescue should be an emergency procedure, we feel that the important part is simply to rescue it. Developing highly specialised centres with ample experience with these techniques and the correct infrastructure could be the next step for achieving a quicker response in rescuing thrombosed accesses. Manual catheter-directed aspiration is an attractive and cost-efficient technique with lower risks and a higher effectiveness than other radiological techniques, particularly in autologous fistulae. The fact that manual catheter-directed aspiration complements angioplasty and stents confirms the need for vascular interventional radiology centres with experience and commitment in the area of vascular access.
Table 1. Type of thrombosed vascular access
Table 2. Duration of use of thrombosed accesses
Table 3. Elapsed time between thrombosis and treatment
Table 4. Elapsed time between thrombosis and treatment
Figure 1.
Figure 2.
Figure 3.
Figure 4.
Table 5. Type of lesions treated (AVF)