ImmuKnow® es un método diagnóstico in vitro que emplea sangre completa del paciente estimulada policlonalmente con fitohemaglutinina y mide la producción de adenosina 5'-trifosfato (ATP) por las células T CD4+. La prueba tiene como objetivo proporcionar una medida objetiva y global de la respuesta inmunitaria celular de cada individuo. El ensayo se diseñó con la idea de monitorizar la inmunosupresión administrada al paciente trasplantado de forma individualizada intentando ayudar a conseguir el equilibrio para evitar un exceso de inmunosupresión con los efectos adversos que conlleva (infecciones, cáncer, etc.) o un defecto de la inmunosupresión con el consiguiente riesgo de rechazo del aloinjerto. La mayoría de los trabajos que han evaluado su utilidad clínica muestra una gran diversidad en cuanto al modo de reclutamiento de los pacientes, el tratamiento inmunosupresor recibido, las variables clínicas analizadas y, sobre todo, el tiempo entre la realización de ImmuKnow® y el evento clínico evaluado. Los datos más consistentes muestran que este ensayo de función de las células T CD4+ resulta útil para predecir el riesgo de infección en trasplantados renales. Sin embargo, no está claro su empleo como marcador de riesgo de rechazo. Por último, dada la enorme variabilidad de la respuesta inmunitaria entre individuos y las publicaciones existentes, se deduce que un valor aislado de ImmuKnow® no tiene capacidad diagnóstica y solo un seguimiento seriado individualizado ayudaría más definitivamente a tomar decisiones clínicas y de cambios en el tratamiento inmunosupresor. Otros aspectos en relación con la aplicación de ImmuKnow® en la rutina clínica, como por ejemplo la periodicidad de la realización de la prueba, precisan estudios prospectivos aleatorizados para una más completa información.
ImmuKnow® is an in vitro diagnosis method that uses patient samples of whole blood polyclonally stimulated with phytohaemagglutinin. It also measures adenosine-5’-triphosphate (ATP) production by CD4+ T cells. The test aims to offer an objective and overall measurement of each individual’s cellular immune response. The assay was designed with the idea of individually monitoring the immunosuppression administered to transplant patients. At the same time, it aims to help achieve a balance as a way of avoiding immunosuppression excess and the associated adverse effects (infections, cancer, etc.) or an immunosuppression defect and the subsequent risk of allograft rejection. The majority of studies that have evaluated its clinical usefulness display great diversity in terms of patient recruitment, the immunosuppressant treatment received, the clinical variables analysed and, above all, the time between performing ImmuKnow® and the evaluated clinical event. The most consistent data show that this assay on CD4+ T cell functioning is useful for predicting the risk of infection in renal transplant patients. However, its use as a rejection risk indicator is unclear. Lastly, given the great variability of immune response amongst individuals and that of existing publications, it can be deduced that the isolated ImmuKnow® value does not have diagnostic capacity and only individual serial monitoring could provide definitive assistance in clinical decision making and immunosuppressant treatment changes. Other aspects of ImmuKnow® application in the clinical routine, such as assay cycles, require randomised prospective studies for more comprehensive information.
INTRODUCTION
This review analyses the information available on a commercial diagnosis method which determines in vitro the intracellular production of adenosine 5'-triphosphate (ATP) in CD4+ T cells. This method is known as ImmuKnow®, uses whole blood of the patient and aims to provide an overall measurement of the cellular immune response of each individual. The method was originally designed as a clinical biomarker of the degree of drug immunosuppression in transplantation. Specifically, three clinical applications have been ascertained: reducing the risk of infection caused by excessive immunosuppression without increasing the risk of cellular rejection, reducing toxicity or undesirable side effects of immunosuppressive drugs, and individualisation of immunosuppression.
Renal transplant success largely resides in the use of potent immunosuppressive drugs that limit the ability of the immune system to reject allografts. During the early post-transplant period, recipients usually receive drugs whose dose is measured in mg/kg body weight until the target blood levels are achieved, which show great variability between individuals.1 In clinical practice the difficult balance between over-immunosuppression with its adverse effects and under-immunosuppression and the risk of rejection should be achieved.2 The survival rate of long-term graft has not changed significantly over the last 20 years, mainly due to lifelong use of immunosuppressant drugs.3,4 The ability to minimise and monitor immunosuppressive therapy individually in each patient would improve the long-term prognosis of renal transplantation and reduce the associated costs.
IMMUNE MONITORING
It is impossible to monitor the overall clinical immunosuppression state of a patient by measuring only one parameter.2 To date, the biomarkers most used in clinical practice have been pharmacokinetics. However, it is the biomarkers related to the pharmacodynamic effects of immunosuppressants which are becoming more important.5 In theory, the routine study of (an) ideal biomarker(s) would identify those patients at risk of acute rejection, infection or cancer, and those patients susceptible to minimising immunosuppression. It would also serve as a fundamental tool in the individual monitoring of patients with under or over-immunisation and could even complement and/or replace pharmacokinetic monitoring. Since the immune system responds quickly and is constantly changing, regular and repeated monitoring of patients seems fundamental to understanding any immune response.
BASICS OF ImmuKnow®
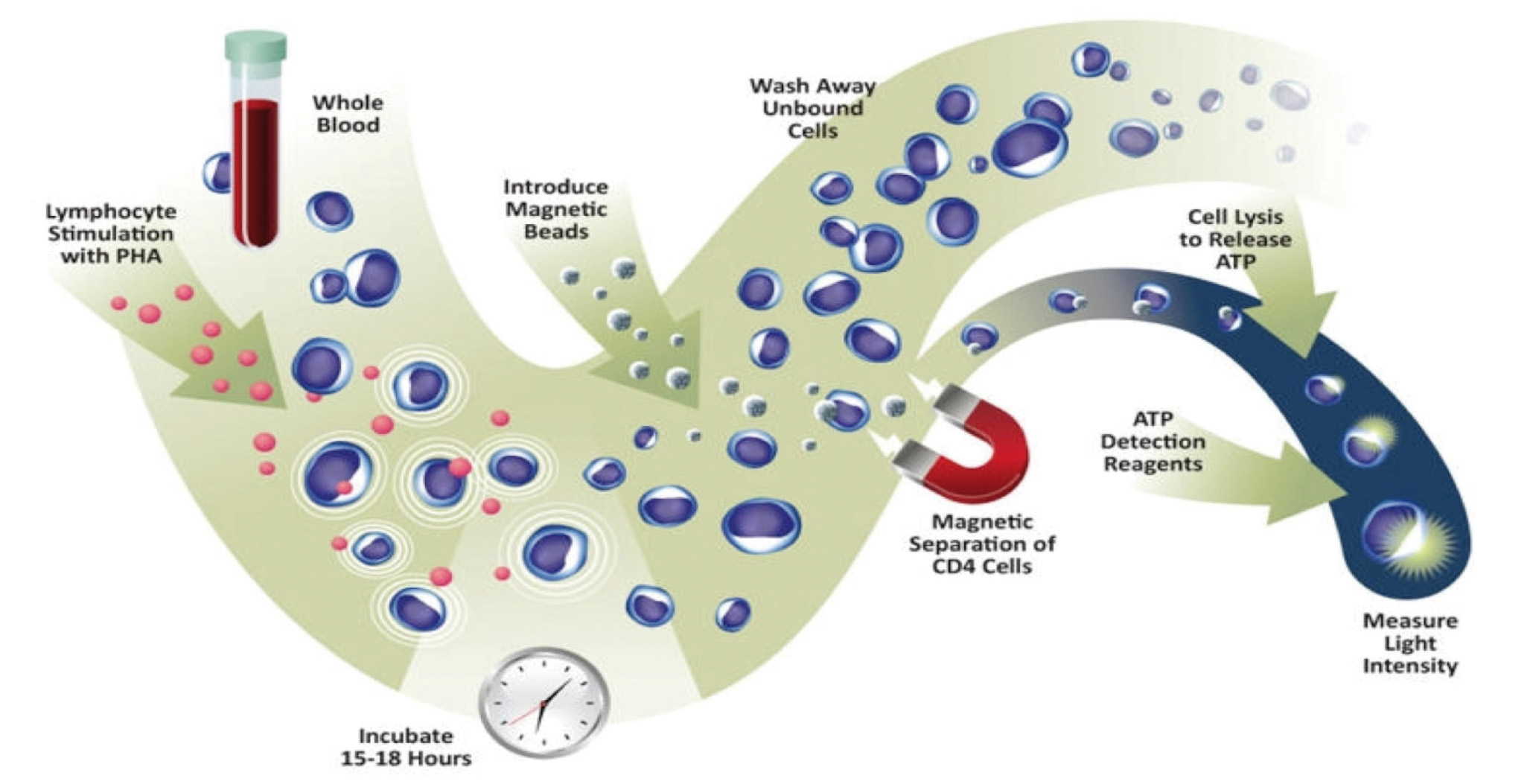
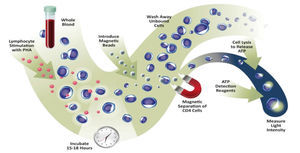
The application of the assay was approved in 2010 by the Food and Drug Administration (FDA) for detection of cellular immune response in patients undergoing organ transplantation and receiving immunosuppressant treatment.6,7 Recently, the assay also exceeded the requirements of the European directive for in vitro diagnosis. Specifically, it measures the ability of CD4+ T cells from a whole blood sample to react to polyclonal stimulus with phytohaemagglutinin (PHA) mitogen (Figure 1). As with immunosuppressive agents used in clinical practice, PHA is not specific to any T cell, and as such, it reflects the ability of any of its subtypes to respond. The method quantifies the amount of ATP produced by CD4+ T cells after overnight incubation with PHA.8 ATP production is an early event in the cell activation process and reflects the response to mitogen stimulation.9
In the case of solid organ transplantation, acute rejection primarily presents in cell activation; however, every day there is increasing evidence of the importance of rejection mediated by antibodies.10 CD4+ T Helper (Th) cells guide the immune response towards a type of cellular response (Th1, Th2, Th17, Tregs, etc.) through the synthesis and secretion of various cytokine combinations, depending on the type of antigen that it finds. Although the purpose of this review is not to investigate multiple immunological mechanisms involved in acute rejection, we must not forget that CD4+ T cells participate in all of them. The importance of this assay is that it provides a functional overall measurement of Th cells and not only the measurement of a metabolite, cell or a drug level.
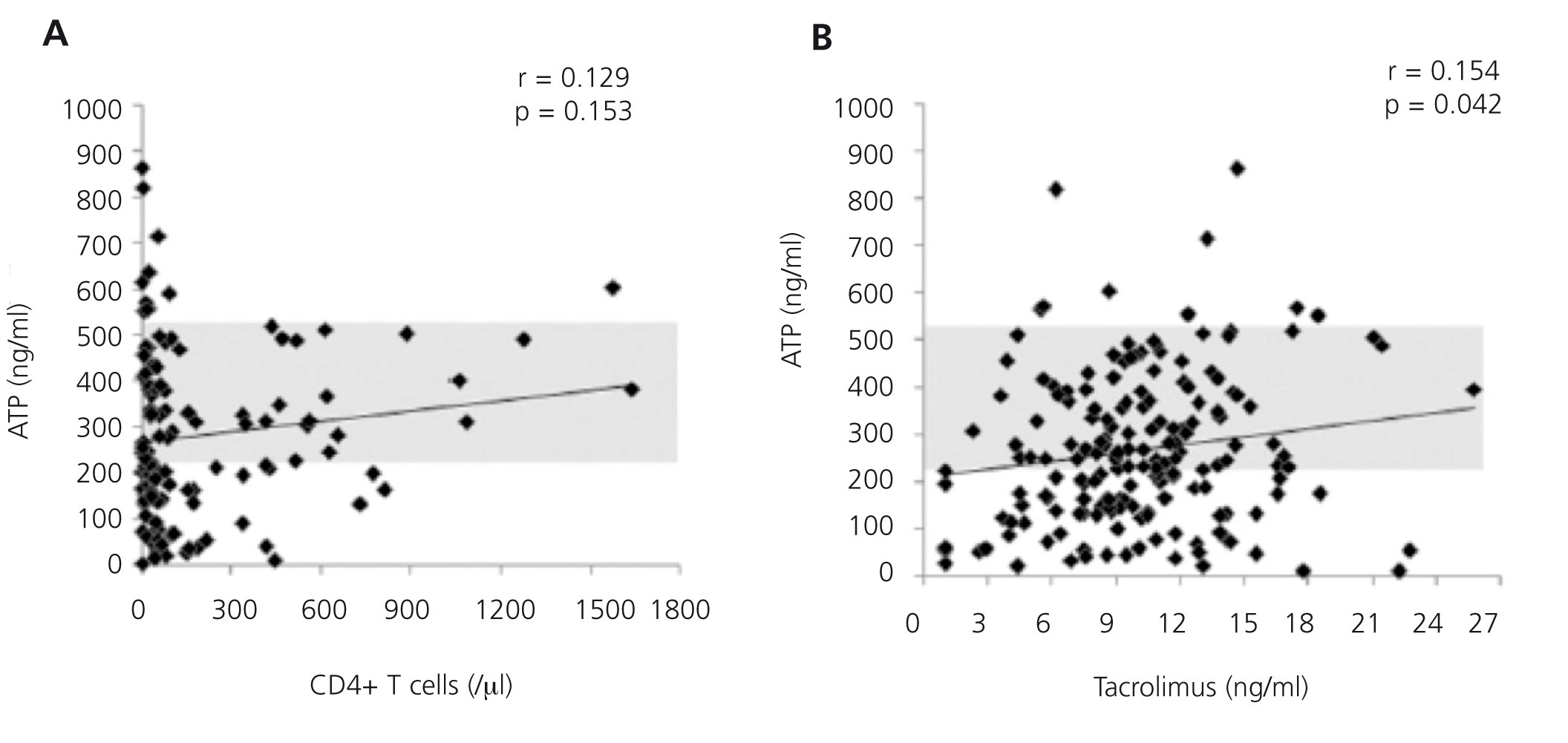
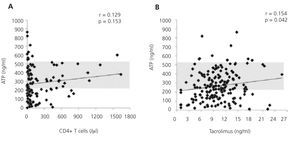
Furthermore, it is a reproducible method that minimises the variability that exists with other cellular assays, detection through flow cytometry of the intracellular cytokine production or cell proliferation measured by the incorporation of tritiated thymidine.11,12 This is achieved because it adjusts the controls to blood volume and not to the number of cells in the assay. It is considered that this situation more reliably reproduces the situation in the bloodstream than the use of a fixed and artificial number of cells. The expected variability of intracellular ATP levels produced between transplant recipients is 11.7%.8 Figure 2A shows the differences in the concentration of ATP produced based on blood volume according to the number of CD4+/ul blood cells in a group of renal transplant recipients undergoing induction treatment with thymoglobulin, followed by triple standard maintenance immunosuppressive therapy.13 Figure 2B also shows the lack of correlation between ATP assay values and tacrolimus13 trough levels. That is, the results of ImmuKnow® are unrelated to the number of CD4+ T cells and blood levels of immunosuppressants. Furthermore, the cellular immunity specific to each patient and therefore, the changes that occur over time provide an individual immune profile that may be a prognosis of phenomena mediated by cellular immunity in relation to excessive or deficient immunosuppression.2,5,14
ImmuKnow® AND ASSOCIATION WITH CLINICAL EVENTS
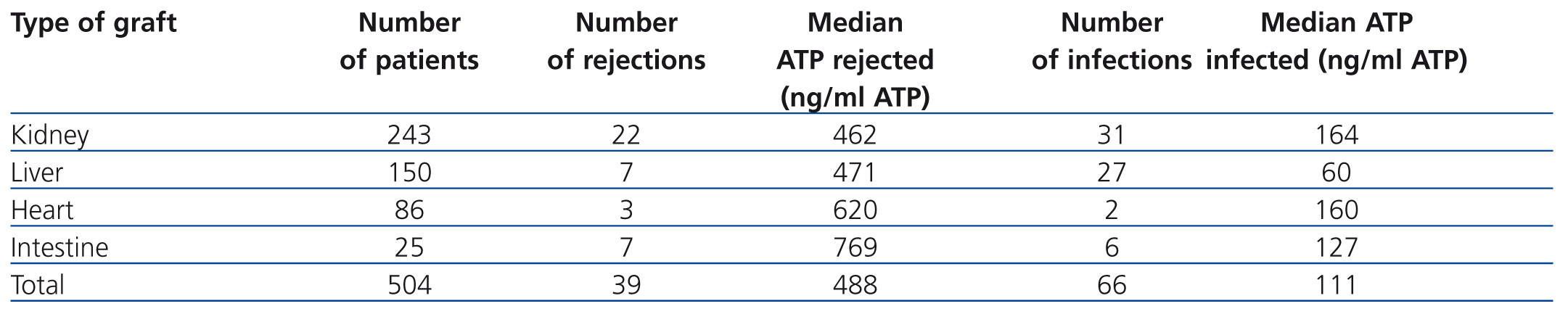
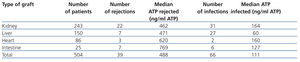
A retrospective multicentre study that included 10 American hospitals compared ImmuKnow® values with the clinical situation of more than 500 recipients of solid organ transplants (kidney, liver, intestine and heart). Table 1 shows how recipients with ImmuKnow® values between 130 and 450ng/ml of ATP had a lower risk of infection or rejection than recipients with values above or below this range, respectively.15 Recipients with an output of 280ng/ml of ATP had a negative predictive value of 96% for both infection and rejection.
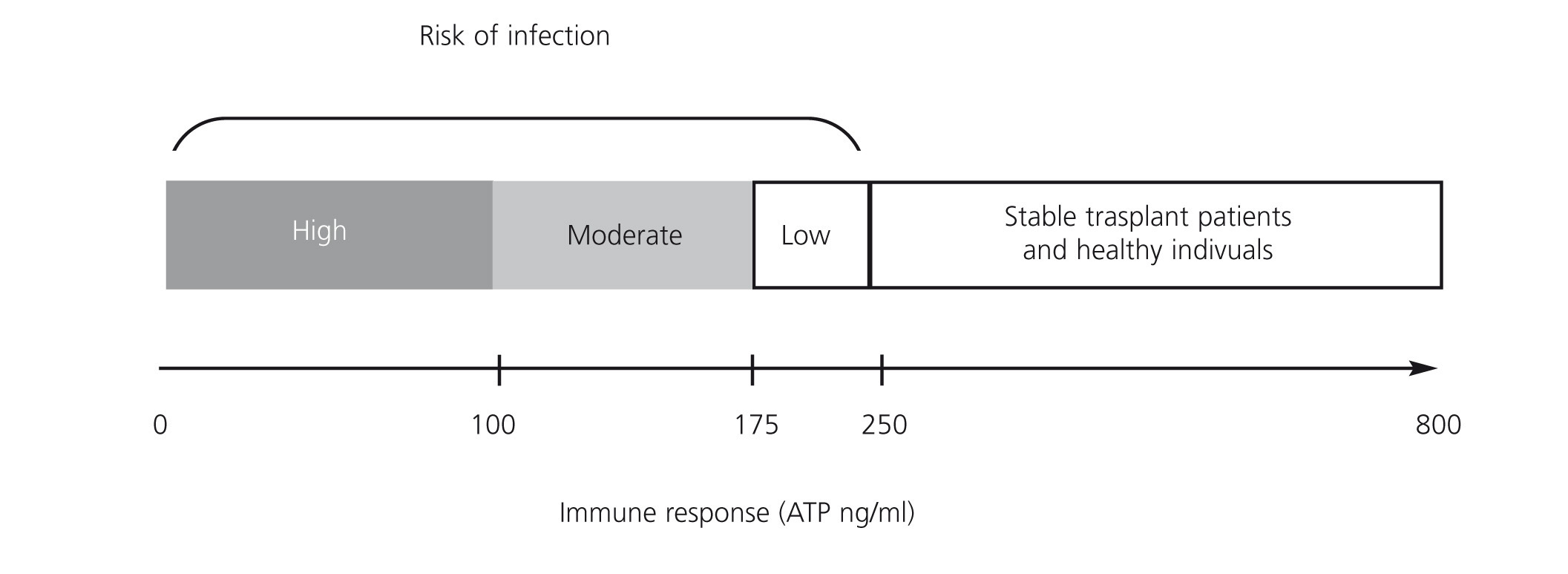
These data have been replicated in other single centre studies in renal transplant patients.13,16-22 The areas under the ROC curve ranged between 0.671 and 0.845 for ATP values of 180ng/ml. The study by Berglund et al.22 on renal, renal pancreas or liver transplant patients showed that severe infections and mortality increased with ATP values below 175ng/ml. The absolute values of the assay are specific to each centre and depend on the specific characteristics of the patients and the immunosuppressive treatment load supplied by each centre. The correlation between intracellular ATP production and clinical episodes shown by most studies is that the latter take place on diagnosis or about 30 days after the episode. The statistical significance of the correlation is lost when the assay is carried out 90 days before the episode.23
Each type of allograft has its own characteristics according to the immune response that it induces. The main risk of renal transplant in particular is infection by the BK virus.23,24 This virus latently infects 95% of the adult kidneys, but can lead to the development of BK nephropathy in immunocompromised patients. It has been estimated that 20-40% of transplant recipients develop viruria with only 12% of viraemia.23 Many centres periodically monitor the presence of viruria and BK viraemia by C-reactive protein (CRP).5,26 Once BK virus is detected in the blood, immunosuppression is reduced empirically or one or more immunosuppressants are eliminated. The risk of rejection is increased if immunosuppression is reduced abruptly or is not restored in time.27
The study by Batal et al.20 showed how measuring with ImmuKnow® in renal transplant patients identified those patients at increased risk of BK nephropathy. BK viraemia patients showed mean ATP levels of 103ng/ml, while those with viruria or negatives had higher figures. Within the viruria group, patients with lower ImmuKnow® levels were associated with higher viral load in urine and, furthermore, these low levels correlating with viral replication eventually resulted in the development of viraemia. In the abovementioned study, of the three patients who developed BK nephropathy, two had low ATP levels (50 and 178ng/ml) 3 and 5 weeks before the development of nephropathy, respectively. The third patient was, in principle, negative for BK, with ATP figures of 206ng/ml. Ten weeks later, the values fell to 106ng/ml and the patient developed BK viraemia.
The usefulness of reducing the risk of immunosuppressive infection with ImmuKnow® has also been described in the post-transplant lymphoproliferative disease caused by the Epstein Barr virus and cytomegalovirus (CMV) infection.19,28-30 A series of 12 renal transplant recipients, admitted due to infections, had very low levels of ATP (range 3-178ng/ml).29 Patients were treated with various maintenance immunosuppression regimens at the time of infection. Treatment consisted of reducing or eliminating one or more of the immunosuppressants that they were receiving, together with antiviral use in some cases. ATP values in the weeks following this treatment increased while viral loads disappeared or were reduced to acceptable levels. There were no episodes of rejection. These results suggest that ImmuKnow® titration could be used to monitor an increase in immunosuppression in order to prevent rejection, once the infection is resolved.
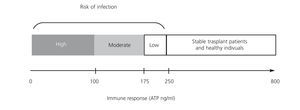
A prospective observational study of 49 renal transplant patients who received induction therapy with basiliximab and maintenance with tacrolimus, mycophenolate mofetil (MMF) and steroids, without selection criteria, determined the production of ATP by ImmuKnow® after 7, 14, 21 and 42 days, and after 3, 6 and 12 months after transplant.19 Seventeen (34.6%) of recipients had infections including: 11 with CMV infection, 2 stomach invasive disease and colitis, 2 with viruria and BK viraemia and two with bacterial infection. All infectious episodes were accompanied by low levels of ATP. CMV replication was predicted from low levels of ATP in 57.1% before the onset of clinical manifestations. Reducing immunosuppression significantly decreased BK replication. Three transplant patients showed clinical and laboratory signs of rejection, which were confirmed by biopsy, but only one showed high levels of ATP. Therefore, the study demonstrated the usefulness of ImmuKnow® in detecting infections and modifying immunosuppression, without involving a risk of cell rejection. The usefulness of ImmuKnow® for predicting the risk of infection was recently demonstrated in two meta-analyses in renal31 and liver32 transplant, and is outlined in Figure 3.
As well as helping predict the risk of infection associated with excessive immunosuppression, ImmuKnow® has also been assessed as an intervention method for preventing toxicities associated with immunosuppression in transplant patients, as with corticosteroids, which can cause high morbidity.33-37 Experimental protocols designed to reduce, avoid or eliminate the use of steroids in transplants have incorporated the ImmuKnow® assay as a monitoring tool. Thus, a clinical trial involving 57 renal transplant patients, of whom 27 were randomised for rapid reduction of the dose of corticosteroids, assessed the utility of ImmuKnow®.38 During the assay, 53% of the control patients were diagnosed with infection and 56% of the latter also had several infections. In contrast, only 22% of randomised patients developed infections and none had repeated infections (p<.05). There was no difference in the rate of rejection between the two groups. In this assay, employing ImmuKnow® to monitor the immune response helped reduce episodes of infection, their duration and the elimination of steroids.
In renal transplantation, but also in other solid organ transplants, it can be useful to reduce, limit or eliminate anti-calcineurin (ACN) immunosuppression drugs.36,39-42 Two macrolides, sirolimus and everolimus and mammalian target of rapamycin (mTOR) inhibitors are used as an alternative to ACN in maintenance immunosuppressive therapy. One study compared ATP levels in stable renal transplant recipients maintained on sirolimus monotherapy with healthy control subjects and it showed how sirolimus treatment induced a significantly lower number of ATP, together with a greater degree of proliferation inhibition in mixed lymphocyte culture and decreased production of interleukin 10 (IL-10).43 Other studies have also shown that very low values of ATP were accompanied by decreased production of IL-10 and increased risk of bacterial infection.44 These results are similar to those of another study in 18 renal transplant patients which analysed the numbers of regulatory T cells and ATP production after conversion to mTOR inhibitors from ACN.41 The regulatory T cell number increased in more than 80% of converted patients and directly correlated with a mean decrease in the production of ATP from 328 to 248ng/ml.
A two-branch assay with 25 kidney-pancreas transplant patients converted from a maintenance treatment with sirolimus, cyclosporine and corticosteroids to another with sirolimus and mycophenolic acid (MPA) used ImmuKnow® to prospectively monitor the immune response.37,40 ATP figures fell slightly and progressively in the 6 conversion months. Subsequently, the numbers gradually stabilised during the remainder of post-conversion follow-up year. In this assay, two patients showed permanent ATP values below 100ng/ml and one patient developed CMV disease. In these patients, the MPA dose was reduced approximately by half and ATP values increased.
A multicentre trial of steroid elimination, randomised in 3 branches with 126 kidney transplant patients, evaluated ImmuKnow® as a biomarker of cellular immunity with other methods, such as ELISPOT, flow cytometry, donor-specific antibodies and HLA compatibility.38 The three branches received thymoglobulin and prednisone as induction therapy until 5 days after transplant. ATP levels were quantified before and after this induction treatment. On average, ATP values decreased from 322 to 172ng/ml of ATP prior to randomisation. Multivariate analysis of the data showed that a value of ATP>375ng/ml was the only variable that correlated (p=.04) with acute cellular rejection and unstable creatinine levels after transplantation.
Our group21 prospectively analysed InmuKnow® in renal transplant recipients in different clinical situations. The assay was used to identify patients at risk of rejection or infection and the information obtained was useful for monitoring immunosuppression. The study concluded that, in stable or infected renal transplant patients with low levels of ATP, the dose of immunosuppression can be reduced safely without increasing the risk of rejection. In stable patients or those with rejection and high ATP figures, the immunosuppression dose may be increased or other immunosuppressants added to prevent immunological damage to the graft, but it seems to provide more useful information in cases of low ATP levels, over-immunosuppression, than in the contrary case.
Another retrospective study monitored the production of ATP before and after renal transplantation in 64 recipients16 and compared it to the type of immunosuppression, doses, blood levels, serum creatinine concentration, white blood cell count, HLA typing, preformed antibodies, adverse effects, infections and rejections. There was no association of the assay with any clinical test, but there were high levels of pretransplant ATP in those with more rejection episodes (8/10), while patients with low ATP numbers had more infections (6/10 P<.001). Patients treated for rejection showed a decrease in ATP figures, 5 days after treatment was begun (P=.002).
The management of immunosuppression has acquired a more complex factor with the appearance of generic ACN and MMF preparations. The narrow therapeutic window of these drugs and the high degree of variability in patients increases the difficulty, since the problem is not just about changing an innovative commercial drug to the generic formula. It is likely that the use of the revised assay here before and during the change to the generic preparation (similar to studies from ACN to imTOR) may serve as a security tool for the clinician and yield results in terms of overall action on cellular immunity in relation to changes in immunosuppressants.
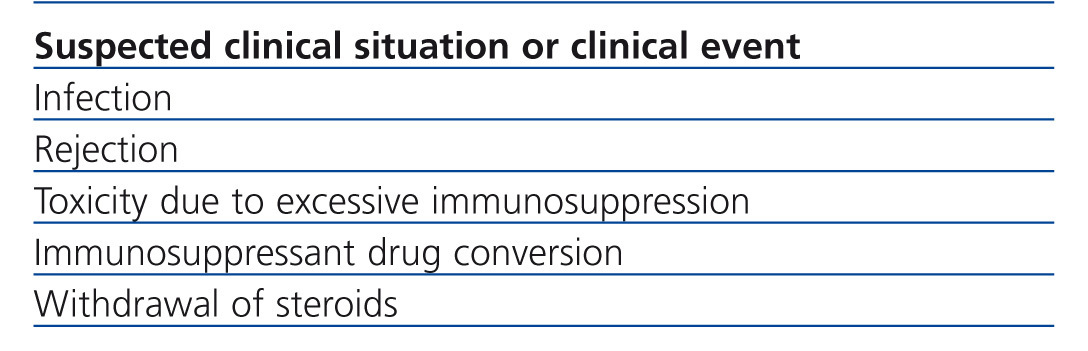
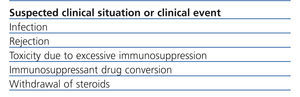
Table 2 summarises the clinical situations in renal transplant where the measurement of intracellular ATP production may be of interest. It should be considered that InmuKnow® is an expensive laboratory method, largely because it is new and because it has not been made available on the market. However, the price of the method is comparable to that of many genetic tests in clinical practice and less than many imaging tests (CT, MRI, etc..) that are requested every day in a hospital. In any case, it should not be considered an open test of the catalogue, but its request must be well established and have clear involvement in the management of renal transplant patient, which will improve the efficiency of the test.
Acknowledgements
We would like to thank Dr. Kowalski for the suggestions and comments he contributed to the drafting of the manuscript.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Key concepts
Table 1. Summary of the number of rejections and infections per transplanted organ and their relationship with detected ATP
Table 2. Possible indications of intracellular ATP measurement in renal transplant patients
Figure 1. Explanatory diagram of assay for measuring in vitro the overall function of CD4+ T blood cells
Figure 2. Effect of the number of CD4+ T blood cells and tacrolimus blood levels on intracellular ATP production.
Figure 3. Proposal of intracellular ATP production level ranges associated with risk of infection in renal transplant in literature published to date