El líquido peritoneal turbio acelular de etiología no infecciosa es una entidad poco frecuente en diálisis peritoneal y se caracteriza por una concentración elevada de triglicéridos en el líquido peritoneal. Las causas más comunes son las neoplasias, las obstrucciones linfáticas, las pancreatitis, los traumatismos y se ha relacionado también con el uso de algunos fármacos, como los antagonistas del calcio. Las series con un mayor número de casos se han comunicado en población asiática. Recientemente hemos diagnosticado en nuestro centro 4 casos de líquido peritoneal turbio acelular relacionado con el uso de antagonistas del calcio. Nos planteamos revisar las características principales de los casos y estudiar la relación del antagonista del calcio con los niveles de triglicéridos en el líquido peritoneal de los pacientes estables en diálisis peritoneal durante el año 2010. De los cuatro enfermos con líquido peritoneal turbio acelular, el 75 % eran varones y el 75 % estaban en tratamiento con manidipino; en todos los casos se resolvió el problema con la retirada del fármaco. Los niveles de triglicéridos medios fueron de 314 mg/dl. Los niveles medios de triglicéridos de 36 pacientes estables de diálisis peritoneal fueron de 8,1 mg/dl, con un intervalo entre 1 y 35 mg/dl. La media de triglicéridos en los pacientes con o sin tratamiento con antagonistas del calcio fue muy similar: 7,81 y 8,6 mg/dl, respectivamente. No se observaron diferencias en relación con el tipo de antagonista del calcio prescrito. En nuestra experiencia, creemos que los antagonistas del calcio deben ser considerados como causa de líquido peritoneal turbio acelular en los enfermos en diálisis peritoneal, en especial el manidipino. No consideramos útil la determinación de triglicéridos en el líquido peritoneal de los enfermos asintomáticos en tratamiento con antagonistas del calcio.
Turbid acellular peritoneal fluid of a non-infectious aetiology is an uncommon entity in peritoneal dialysis and is characterised by a high concentration of triglycerides in the peritoneal fluid. The most common causes include cancer, lymphatic obstructions, pancreatitis, trauma, and even the use of certain medications such as calcium antagonists. The largest studies concerning this entity have been carried out in patients of Asian descent. We recently diagnosed 4 cases of turbid acellular peritoneal fluid at our institution in relation to the use of calcium antagonists. We reviewed the primary characteristics of these cases and examined the relationship between the use of calcium antagonists and triglyceride levels in the peritoneal fluid of stable patients on peritoneal dialysis during 2010.
Of the four patients with turbid acellular peritoneal fluid, 75% were male and 75% were on treatment with manidipine; in all cases, the issue was resolved by suspending medication. Mean triglyceride levels were 314mg/dl. Mean triglyceride levels in 36 stable patients on peritoneal dialysis were 8.1mg/dl, with a range of 1-35mg/dl. Mean triglyceride levels in patients with and without calcium antagonist treatment were very similar, at 7.81mg/dl and 8.6mg/dl, respectively. We did not observe significant differences in terms of the type of calcium antagonist prescribed.
In our experience, we believe that calcium antagonists should be considered as a cause of turbid acellular peritoneal fluid in patients on peritoneal dialysis, in particular manidipine. We do not find it useful to determine triglyceride levels in the peritoneal fluid of asymptomatic patients on treatment with calcium antagonists.
Turbid acellular peritoneal fluid (TAPF) of non-infectious aetiology is an uncommon entity in peritoneal dialysis (PD). This condition is characterised by cloudy peritoneal fluid with a high content of lymphatic fluid and triglycerides (TG), without the presence of leukocytes. The most frequent causes are cancers such as lymphomas, cirrhosis, lymphatic obstructions, pancreatitis, trauma, nephrotic syndrome, and even the use of certain medications.1-3 The most commonly reported drugs in association with this condition are dihydropyridine calcium antagonists (CA), such as lecarnidipine,4,5 manidipine, and nifedipine.6,7 The largest studies thus far carried out in relation to this entity have dealt with patients of Asian descent,4,6,7 and all reported significant TG levels increases in TAPF.4,6,7
During the 2008-2010 period, we diagnosed 4 cases of TAPF related to the use of CA at our institution. Based on these cases, we established the following objectives: to review cases of TAPF, their main characteristics, and their relationship with the use of CA, and to examine the relationship between treatment with CA and TG levels in peritoneal fluid (PF) in prevalent patients on PD.
MATERIAL AND METHOD
In 2010, TG levels were measured in 24-hour peritoneal effluent for all clinically stable prevalent patients on PD. We reviewed anti-hypertensive treatment prescriptions and the use of CA in these patients. We also closely reviewed the four cases of TAPF diagnosis at our institution, the complementary tests carried out, and patient evolution.
CLINICAL CASES AND RESULTS
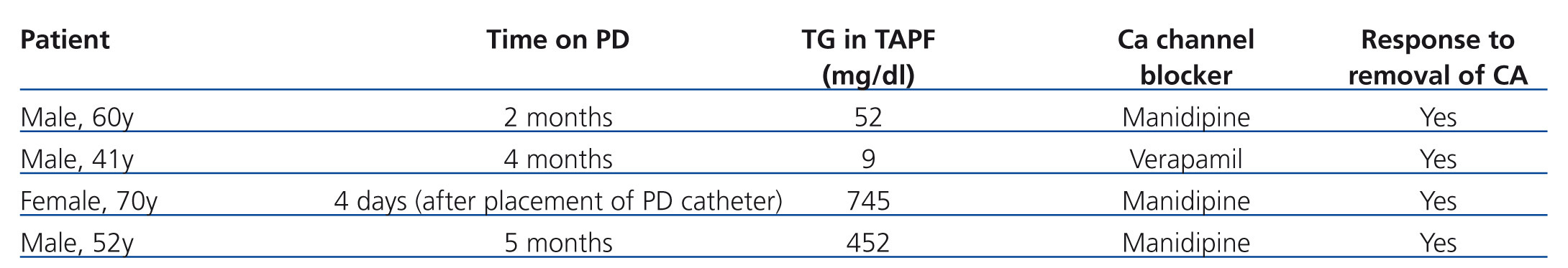
Case 1: 60-year-old male with chronic kidney disease (CKD) secondary to nephroangiosclerosis, who developed TAPF two months after commencing PD. Laboratory analyses revealed normal values for amylase levels and liver parameters. Microbiological cultures of PF for bacteria, fungal contamination, and mycobacteria were all negative. Malignant cell cytology tests were also negative. An abdominal computed axial tomography was normal. Manidipine was finally suspended, resulting in immediate alleviation of the symptoms.
Case 2: 41-year-old male with CKD secondary to diabetic nephropathy who developed TAPF four months after starting PD. The patient was on treatment with verapamil. Complementary tests and peritoneal fluid cultures were all negative. TG levels as measured in this patient were 9mg/dl, but the PF returned to normal as soon as medication was suspended. We believe that the cause of the cloudy fluid was treatment with CA, although we could not demonstrate high TG levels.
Case 3: 70-year-old female with CKD of an unknown aetiology who developed TAPF four days after the laparoscopic placement of a PD catheter (Figure 1). The initial suspicion was one of a lymphatic trauma following catheter placement, but removal of manidipine from the treatment regimen resolved the issue.
Case 4: 52-year-old male with chronic renal failure secondary to nephroangiosclerosis who developed a genital leak and TAPF 5 months after starting PD. The patient was on treatment with manidipine, and the PF returned to normal upon suspension of the medication.
It is interesting to note that all patients were on treatment with CA as part of their regular prescription prior to the start of PD.
The main characteristics of each patient and TG levels are summarised in Table 1.
In 2010, a total of 42 patients received PD, 69% of which were male, with a mean age of 66 years. In addition, 69% were on manual peritoneal dialysis techniques and 45% were on treatment with some type of CA (50% nifedipine, 31% amlodipine, 13% manidipine, and 6% diltiazem). We obtained 24-hour PF TG levels in 36 patients. Mean TG levels were 8.1mg/dl, with a range of 1-35mg/dl, and mean TG levels were very similar between patients with and without treatment with CA, at 7.81mg/dl and 8.6mg/dl, respectively. We did not observe significant differences in TG values based on the type of CA administered, although manidipine produced the highest levels (Table 2).
DISCUSSION
We present a four-case series of TAPF associated with treatment with CA, the largest such study reported in Europe. The closest such study from the continent came from Turkey, in which three patients treated with lecarnidipine were analysed.5 Recently, an isolated case was reported in Spain in association with the administration of manidipine.8
We also describe the first case of TAPF associated with verapamil; although a recent publication reported a case associated with a non-dihydropyridine CA, diltiazem.9
We also observed a high incidence of cases related to the use of manidipine. In our study, 75% of patients with TAPF were on treatment with this drug. These results are in accordance with those from the study by Yoshimoto et al. in 1998, in which 15 Japanese hospitals participated, with a total of 251 patients on PD. These authors describe 19 patients with TAPF associated with treatment with CA, of which 78% were on treatment with manidipine. A total of 42% of all patients on PD who received this drug developed TAPF.7
Cases have also been published in Taiwan and Turkey of patients on treatment with lecarnidipine, in which 58% of individuals receiving this medication developed TAPF.4,5 during this period, we did not observe any patients on treatment with lecarnidipine. Isolated cases of TAPF have also been reported in association with treatment with benidipine, nisoldipine, and nifedipine.7 In our experience, we have not observed any cases of TAPF in association with nifedipine, despite prescribing this treatment in 50% of patients.
As regards the TAPF diagnosis, it is important to carry out a differential diagnosis in order to rule out other potentially severe causes. With 20 years of experience at our PD unit, we have never recorded a case of TAPF associated with cancer or pancreatitis. The primary indicator of a possible case of TAPF associated with CA treatment is an increase in TG concentrations in PF. The mean TG in the PF from the lecarnidipine study was 128.4mg/dl.4 In a recently published case of TAPF associated with manidipine, mean TG was 314mg/dl, higher than the standard values reported in the medical literature.
In our analysis of TG concentrations in normal PF in our patients, we obtained a mean value of 8.1mg/dl, with a range of 1-35mg/dl, lower than the mean levels reported for TG in TAPF, which tend to surpass 100mg/dl.4,8 We also observed similar TG values in patients on treatment with CA and those not on CA. As such, the use of CA in patients with normal PF does not produce high TG levels in PF.
In an effort to describe the possible causes of this phenomenon, several authors have mentioned the action of CA on the peritoneal membrane, resulting in increased perfusion and peritoneal function,10,11 which is attributed to the reduction produced by CA in inflammatory cytokines.12,13 Third-generation CA, such as manidipine, lercadipine, amlodipine, nivaldipine, and efonidipine, are capable of blocking both type L and type T calcium channels, the latter being active only during cell proliferation. These are very lipophilic molecules,13,14 which may act in the smooth muscle cells of the lymph vessels within the digestive tract, thus hindering lymph return. The geographic distribution of several studies carried out in Asia and very few in the rest of the world might suggest a genetic factor and ethnic predisposition,4,6,7 although the causes have yet to be elucidated. All of our patients were Caucasian.
To conclude, we believe that CA should be considered as a cause of non-infectious TAPF in patients on PD. In our experience, the CA that most frequently produced TAPF was manidipine, and lecarnidipine should also be used with caution based on the results described in Asian studies. Taking into account our results, we do not believe that it is necessary to measure TG in the PF of patients on treatment with CA, since there were no differences observed between patients based on treatment with or without CA, or based on type of CA used. The removal or limitation of these drugs in patients on PD is not justified solely based on this phenomenon. If an episode of TAPF occurs, removing the implicated drug or switching to a less lipophilic CA should be considered.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the content of this article.
Table 1. Summary of the four cases of turbid acellular peritoneal fluid
Table 2. Mean triglyceride levels according to type of calcium antagonist administered
Figure 1. Turbid acellular peritoneal fluid from a patient treated with manidipine