Los compuestos que contienen magnesio constituyen una aportación muy prometedora como captores del fósforo en el control de la hiperfosfatemia en el paciente con enfermedad renal crónica (ERC). Sin embargo, el efecto del magnesio en pacientes con ERC no está aún claro. Este artículo tiene como objetivo mejorar el conocimiento acerca del papel fisiológico del magnesio en la población general y en el paciente con ERC en particular. La prevalencia de la hipomagnesemia es elevada en la población general y especialmente en los pacientes ingresados en las Unidades de Cuidados Intensivos, y en muchos casos pasa inadvertida. El déficit de magnesio aumenta el riesgo de sufrir algunas enfermedades, como la diabetes tipo 2, la hipertensión y la arteriosclerosis. Por otra parte, la hipermagnesemia moderada parece tener efectos beneficiosos sobre la calcificación vascular y la mortalidad en pacientes con ERC. Además, se ha observado que la hipermagnesemia se asocia a niveles más bajos de hormona paratiroidea en estos pacientes, aunque los resultados sobre el hueso son controvertidos. Asimismo, la reducción del magnesio sérico se asocia a bajos índices de volumen óseo, osteoporosis y calcificación vascular. En los pacientes en diálisis, los niveles séricos de magnesio dependen principalmente de la concentración en el líquido de diálisis. A largo plazo, la administración de magnesio a los pacientes en diálisis podría retrasar la calcificación arterial y mejorar la supervivencia, pero se necesitan estudios prospectivos aleatorizados potentes para confirmar estos efectos. Puesto que hay un estudio aleatorizado prospectivo reciente que demuestra que un captor del fósforo que combina carbonato de magnesio y acetato cálcico fue igual de efectivo que el compuesto polimérico sevelamer ClH con similar perfil de tolerancia, procede examinar y poner al dia el papel del magnesio en los pacientes con ERC.
Magnesium containing compounds present promising oral phosphate binders for the treatment of hyperphosphataemia in patients with chronic kidney disease (CKD). However, the impact of magnesium in CKD patients still remains unclear in clinical routine practice. Therefore, this publication provides a practicable overview of knowledge about the physiological role of magnesium in general and in particular in CKD patients. Prevalence of hypomagnesaemia is high in the general population and especially in intensive care unit patients, but often not being detected. Magnesium deficiency increases the risk for several diseases, like diabetes mellitus type 2, hypertension and atherosclerosis. Moderate hypermagnesaemia, however, seems to have beneficial effects on vascular calcification and mortality rates in CKD patients. On the other hand, higher serum magnesium levels are reported to be linked to lower PTH levels and results on the effects on bone are controversial. In addition, low magnesium levels are associated with low bone mass, osteoporosis and vascular calcification. In dialysis patients serum magnesium levels are dependent mainly on the dialysate magnesium concentration. To confirm the potential delay of arterial calcification and improved survival outcomes by long-term intervention with magnesium powered randomized studies are required in dialysis patients. Since a recent trial revealed that a phosphate binder containing a combination of magnesium carbonate and calcium acetate was as effective as the polymer-based agent sevelamer hydrochloride and had an equally good tolerability profile, it is time for a re-examination of the role of magnesium in CKD patients.
INTRODUCTION
Magnesium, the fourth most abundant cation in the body, plays an important role in numerous enzymatic reactions, transport processes and synthesis of proteins, DNA and RNA. In contrast to its physiological role, the clinical importance of magnesium is often underestimated. Disorders of magnesium are hardly mentioned in most educational books of medicine. Furthermore serum magnesium concentrations are not measured routinely in hospitalized patients and thus most magnesium abnormalities are remaining undetected (Whang et al. 1990). During the last 20 years substantial knowledge has been accrued about magnesium and new avenues have been opened for patients. The present publication gives an overview about magnesium metabolism and disorders in magnesium balance, especially in chronic kidney disease (CKD) patients. The role of magnesium-containing phosphate binders in CKD patients is re-examined, since their use offers a chance to circumvent problems associated with other agents of this class. This review represents a brief compendium of the more detailed magnesium supplement written by experienced scientists and clinicians (Luft 2012).
MAGNESIUM METABOLISM AND PHYSIOLOGY
Magnesium fulfils various intracellular functions. It stabilizes enzymes in many ATP generating reactions, antagonizes calcium in muscle contraction, modulates insulin signal transduction and cell proliferation, and is important for cell adhesion and membrane transport (Jahnen-Dechent et al. 2012). About 99% of total magnesium is located in bone, muscles and non-muscular soft tissue. Extracellular magnesium accounts for about 1% and is primarily found in serum and red blood cells (Elin 1988).
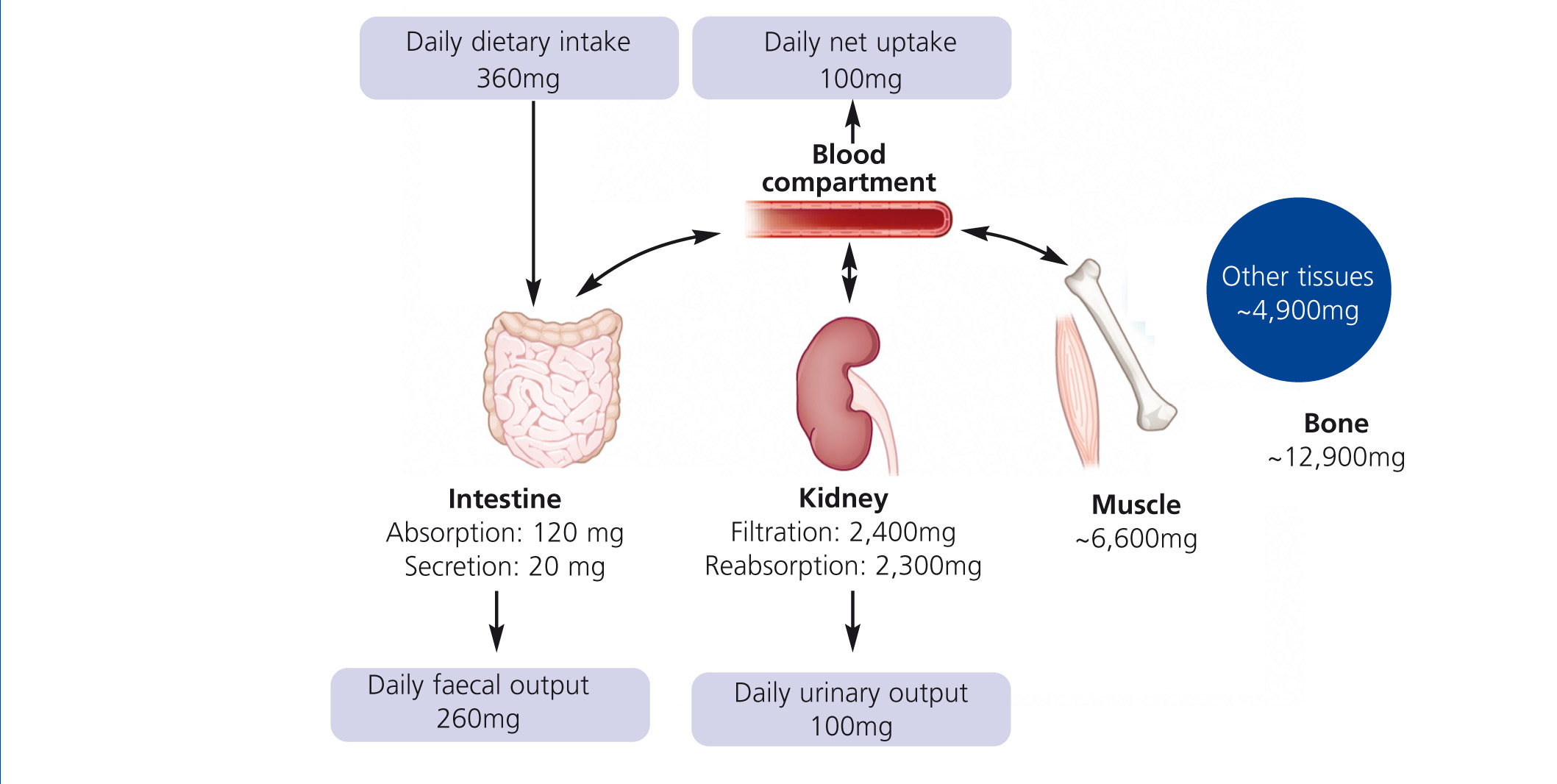
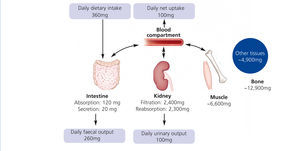
Humans have to consume magnesium regularly to prevent a deficiency. The Institute of Medicine recommends 310–360 mg and 400–420 mg for adult women and men, respectively. Intestine, bone and kidneys maintain magnesium homeostasis (Figure 1): Magnesium is absorbed in the gut, stored in bone and muscles and excreted by the kidneys. Intestinal absorption is dependent on magnesium status: The lower the magnesium level, the more is absorbed. However, regulation of intestinal magnesium uptake is highly complex and still not fully understood. The majority of magnesium is absorbed by a passive paracellular mechanism, which is driven by the electrochemical gradient (Jahnen-Dechent et al. 2012). A minor but important fraction of magnesium is actively transported via transcellular channels (de Baaij et al. 2012). Besides intestinal uptake renal excretion is crucial in maintaining magnesium balance. In kidneys a mechanism comparable to the intestinal uptake regulates magnesium reabsorption. Up to 90% of the filtered magnesium is reabsorbed in a passive paracellular fashion. Fine-tuning takes place in a dedicated part of the nephron, the distal convoluted tubule. Here, magnesium-specific transporters carefully keep magnesium reabsorption within a sizeable range: renal excretion of the filtered load may vary from 0.5%-70%. In moderate CKD (stage 1-3) loss of renal function is compensated by an increased fractional excretion of magnesium, while this mechanism fails in advanced CKD resulting in hypermagnesaemia. In dialysis patients, serum magnesium levels mainly depend on dialysate magnesium and dietary intake.
The most common test for the assessment of the magnesium status is the measurement of serum magnesium levels – a practicable and inexpensive test. However, it should always be kept in mind that serum magnesium levels do not reflect the total body magnesium status.
Reference ranges for serum magnesium levels are 0.65–1.05 mmol/L for total magnesium and 0.55–0.75 mmol/L for ionized magnesium in adult blood serum (Tietz 1990; Mai-Zurawska 1994). The prevalence of hypomagnesaemia in hospitalized patients is high (9-65%; Hashizume et al. 1990) especially in patients on postoperative intensive care units (Chernow et al. 1989). In the general population, hypomagnesaemia frequently occurs in patients with diabetes, chronic gastrointestinal diseases, alcoholism and after the use of certain types of drugs. A small but interesting group of patients have a hypomagnesaemia that is the result of genetic mutations. Specifically, mutations in genes that encode ion transporters in the distal convoluted tubule can explain many familial forms of hypomagnesaemia (de Baaij et al. 2012). In particular mutations in the TRPM6 magnesium channel have been shown to account for the largest part of the genetic forms of hypomagnesaemia. Hypermagnesaemia is often associated with undetected renal impairment and excessive oral administration of magnesium-containing drugs (e.g. laxatives or antacids) or with advanced CKD (Jahnen-Dechent et al. 2012; Xing et al. 2001). Clinical signs of both hypo- and hypermagnesaemia are non-specific, can be similar or even absent. Signs include loss of appetite, fatigue and weakness, later on, as magnesium deficiency worsens, numbness, cramps, seizures, personality changes, arrhythmias and coronary spasms. Severe hypermagnesaemia might lead to depression or loss of deep tendon reflexes, hypotension, gut paralysis and ECG-changes. At very high and very low serum magnesium levels severe neuromuscular dysfunction, hypotonia and even pseudoparalysis, respiratory depression, areflexia and coma may develop.
SERUM MAGNESIUM AND DISEASE RISK
Various epidemiological studies demonstrated associations between low serum magnesium levels and an increased risk for metabolic syndrome, type 2 diabetes mellitus (T2DM), hypertension and atherosclerosis (Geiger et al. 2012). However, the use of magnesium as a therapeutic agent is only indicated for pre-eclampsia and specific forms of arrhythmias (figure 2). Cardiovascular disease and T2DM are common comorbidities of CKD and therefore the role of magnesium in these diseases is discussed in more detail.
Hypomagnesaemia was found in 14-48% of T2DM patients versus 3-15% of the healthy control group and has been implicated in adversely affecting several diabetic complications (Pham et al. 2007). A retrospective study with 550 T2DM patients without known kidney disease revealed, that lower magnesium levels were associated with further deterioration of renal function. Patients with higher serum magnesium levels (between 0.82–1.03 mmol/L) had the slowest progression and the best glycaemic control. Therefore these serum levels were suggested as target levels for diabetic patients (Pham et al. 2005). A meta-analysis of seven studies (n=286,668) found, that an increased magnesium intake of 100 mg/day is inversely associated with the incidence of T2DM (Larsson et al. 2007). The authors suggested that magnesium-rich food might reduce the risk for T2DM. Whether patients with established T2DM benefit from additional magnesium supplementation was evaluated in a meta-analysis of nine randomized trials enrolling 370 T2DM patients (Song et al. 2006). Although dosage and inclusion criteria varied and the numbers of patients in single studies were relatively small, magnesium supplementation (median dose of 15 mmol/day) used as an adjunct therapy for 4–16 weeks was found to be significant regarding lowering fasting glucose levels (Song et al. 2006). Thus, daily magnesium supplementation seems to be beneficial in pre-diabetic and T2DM patients.
Also, many cardiovascular disorders are associated with low magnesium levels. The authors of a recent epidemiological study (n=212,157) found that low magnesium levels predicted cardiovascular and all-cause mortality (Reffelmann et al. 2011). They also showed that low serum magnesium concentrations − regardless of other risk factors − were associated with long-term gain of left ventricular mass, a significant predictor for adverse cardiovascular events.
Hypomagnesaemia has also been linked to high blood pressure. In the ARIC study (n=15,248) serum magnesium levels were inversely related to systolic blood pressure (Ma et al. 1995). Further, a meta-analysis of 20 randomized studies (n=1,200) revealed that magnesium supplementation is associated with significant dose-dependent blood pressure reductions (Jee et al. 2002). However, other more recent trials failed to demonstrate blood-pressure-lowering effects of magnesium supplementation alone but suggest that they enhance the effect of antihypertensive medications (Dickinson et al. 2006; Rosanoff 2010).
Atherosclerosis is a well-known risk factor for cardiovascular disease, potentially triggering myocardial infarction and stroke. The pathogenesis of atherosclerosis is complex and like endothelial dysfunction and hyperlipidaemia, hypomagnesaemia has been identified as a major risk factor. According to the follow-up of the ARIC study (n=13,922), patients with the lowest serum magnesium level had the highest risk for coronary artery disease (Liao et al. 1998). Furthermore, Ascherio et al. (1998) found a negative association between dietary magnesium intake and risk of stroke in a prospective study (n=43,738). Inverse associations were all stronger in hypertensive than normotensive men and were not materially altered by adjustment for blood pressure.
Hypomagnesaemia also seems to be involved in the pathogenesis of ischaemic heart disease by altering lipoprotein composition and modifying post myocardial arrhythmia. For this reason, magnesium therapy has been extensively studied in the context of acute myocardial infarction in various clinical trials (Woods et al. 1994, Geiger et al. 2012). However, the conclusion after the last clinical trials (ISIS 4 Collaborative Group 1995) was that magnesium sulphate cannot be generally recommended for the routine administration after acute myocardial infarction.
MAGNESIUM IN CHRONIC KIDNEY DISEASE (CKD)
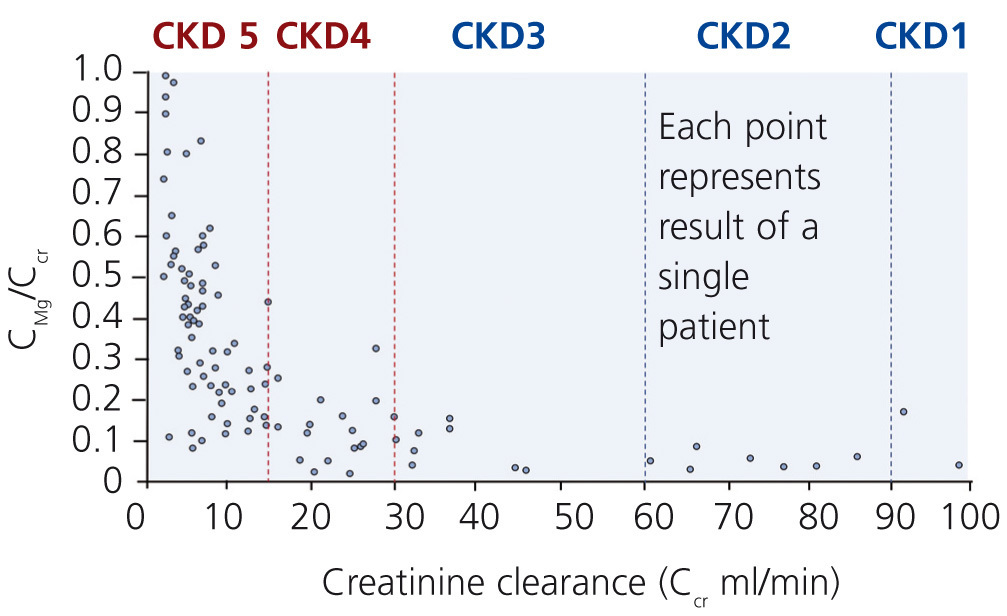
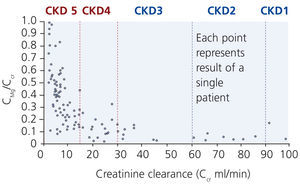
Although excretion of magnesium via the kidney is highly adaptable, this ability deteriorates when renal function declines significantly (Cunningham et al. 2012). In moderate CKD (stage 1-3), an increase in fractional excretion compensates for the loss of renal function such that magnesium levels are maintained within the normal range (Coburn et al. 1969). Interestingly, there are differences in diabetics and non-diabetics. A significant correlation between a low creatinine clearance and a high serum magnesium was found in non-diabetics but not in diabetics. In these, serum magnesium levels were significantly lower despite reduced creatinine clearances (p<0.001; Dewitte et al. 2004).
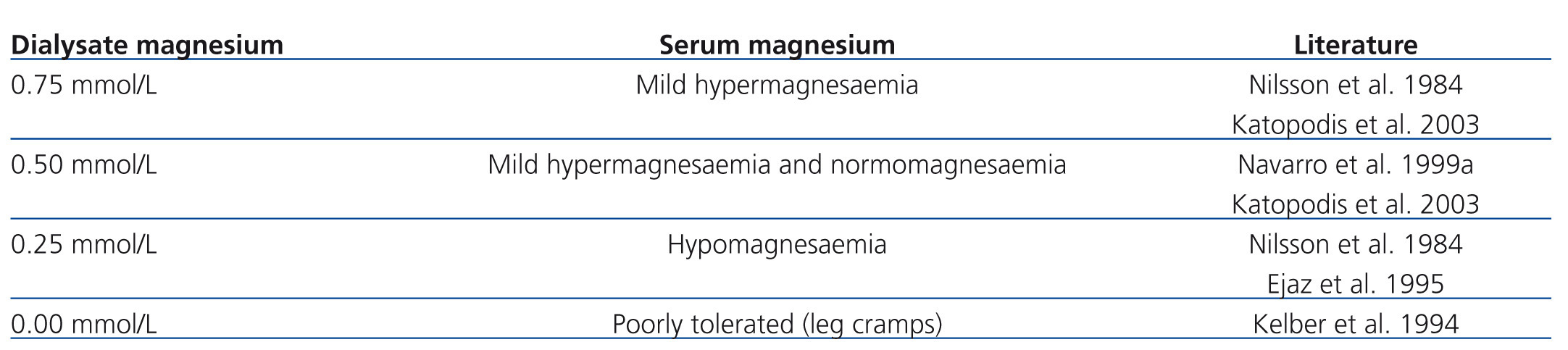
In more advanced CKD (stage 4-5) renal compensatory mechanisms become inadequate. Hypermagnesaemia develops frequently in patients with creatinine clearances less than 10 mL/min (figure 3). In dialysis patients, magnesium concentrations are dependent mainly on dialysate magnesium. Magnesium crosses the dialysis and peritoneal membrane readily. Various magnesium dialysate concentrations have been investigated in patients undergoing haemodialysis (HD) or peritoneal dialysis (PD) (Table 1). Magnesium dialysate concentrations of 0.75 mmol/L may cause mild hypermagnesaemia, whereas a concentration of 0.25 mmol/L mostly causes hypomagnesaemia. Results for the magnesium dialysate concentration of 0.5 mmol/L are less consistent but magnesium levels are mostly within the normal range. The inconsistent results of several studies suggest that other factors, such as nutrition and magnesium supplements (e.g. antacids), may also play an important role in determining serum magnesium levels in dialysis patients.
One important subject in CKD patients is the relationship of magnesium with parathyroid activity. Multiple abnormalities contribute to the development of secondary hyperparathyroidism (sHPT), a common complication of CKD. Serum calcium, calcitriol, fibroblast growth factor 23 (FGF23) and serum phosphate have key roles in regulating parathormone (PTH) synthesis and secretion (Cunningham et al. 2012). Calcium is the dominant activator of the calcium sensing receptor (CaSR), but magnesium also activates CaSR, though with a potency 2–3fold less than calcium. Even though, serum magnesium levels may have a regulatory role in PTH secretion.
The relationship between serum magnesium and PTH levels in patients undergoing HD or PD were investigated in several studies (Cunningham et al. 2012). Navarro et al. (1999a+b) found a significant inverse relationship between serum magnesium and PTH levels (p<0.001) in 110 HD as well as 51 PD patients using 0.5 mmol/L and 0.75 mmol/L magnesium dialysate, respectively. However, most other studies used concomitant changes of magnesium and calcium dialysate concentrations (Wei et al. 2006). Therefore result interpretations are difficult and further research is necessary to elucidate the influence of magnesium on PTH levels independent of calcium changes.
55% of the body’s magnesium content is found in bone, the largest magnesium store in the human body. But magnesium only represents a tiny proportion (below 1%) of the total bone mineral that mainly consists of calcium and phosphate in form of hydroxyapatite. Studies about magnesium concentrations in the bone of CKD patients revealed highly variable results indicating that several factors influence the uraemic bone metabolism. The role of magnesium in the pathogenesis of renal bone disease has been studied in recent years. Magnesium seems to be crucial for the regulation of osteoblast and osteoclast activity and bone remodelling. Magnesium deficiency in rat caused impaired bone growth, osteopenia and skeletal fragility (Rude et al. 1999). Epidemiological studies have linked insufficient magnesium in the diet to low bone mass and osteoporosis (Rude et al. 2009). However, many other factors, like vitamin D status, PTH level, calcium and phosphate concentrations and metabolic acidosis, contribute also to bone metabolism and the exact role of magnesium needs to be investigated in more depth.
MAGNESIUM AND VASCULAR CALCIFICATION IN CKD
Cardiovascular disease is the leading cause of death in both CKD and PD/HD patients (Foley et al. 1998). Patients with CKD undergoing dialysis have 2- to 5-fold more coronary artery calcification than age-matched individuals. Calcifications in the intimal and medial vessel layer are major direct or indirect contributors to cardiovascular disease and mortality in CKD patients.
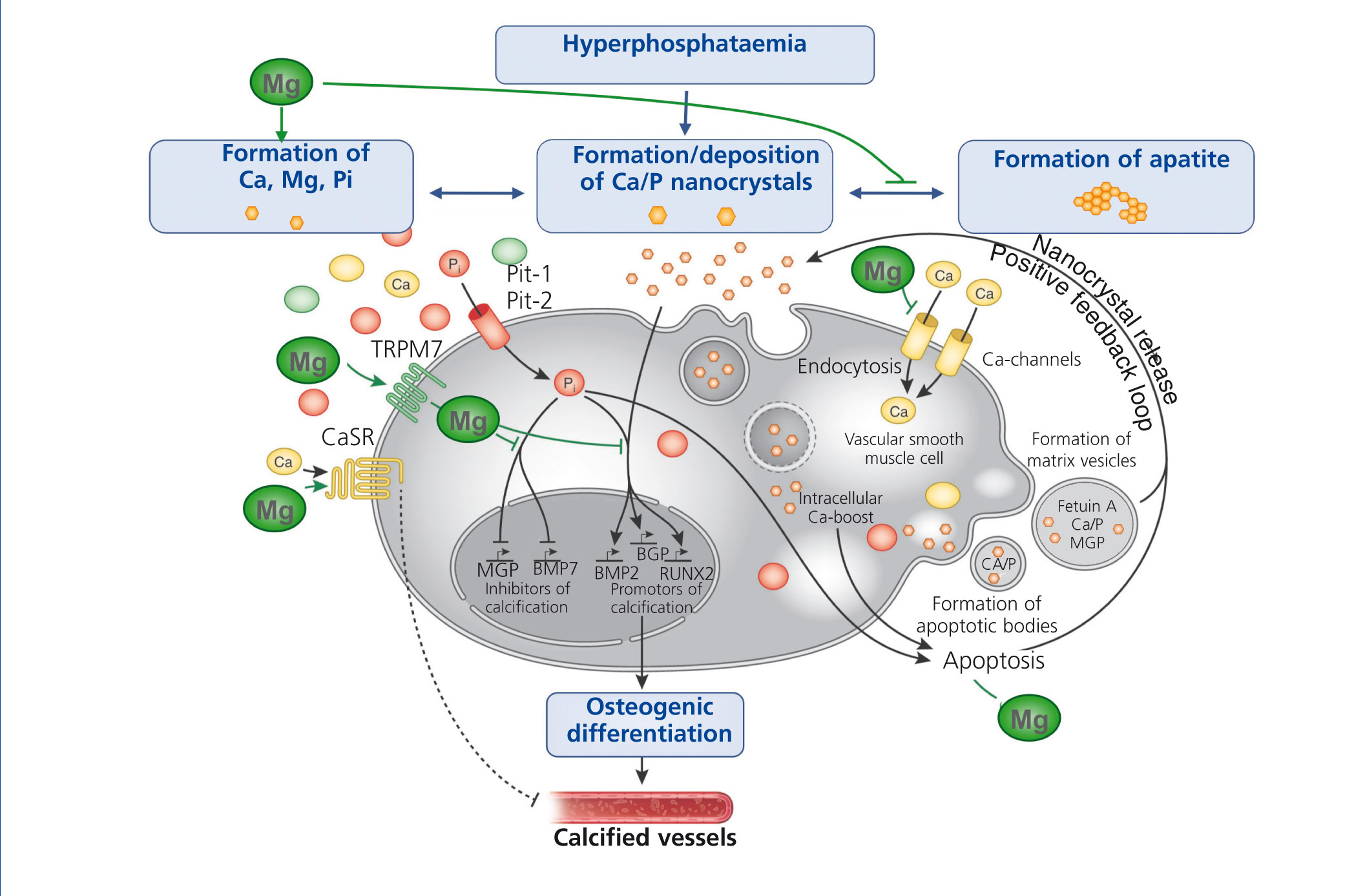
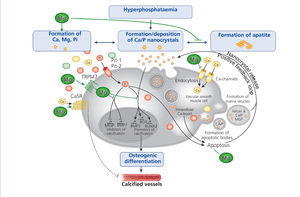
The progressive loss of kidney function is accompanied by elevated FGF-23 levels, a decrease in phosphate excretion and a dysregulation of bone metabolism. Disturbances in mineral and bone metabolism clearly promote vascular calcification. The pathogenesis of vascular calcification is a cell-mediated and actively regulated process that closely resembles the formation of normal bone tissue (Massy et al. 2012). A central role play vascular smooth muscle cells (VSMCs) that compose the medial layer of the vessel wall and converse into osteoblast-like cells – a phenotype that is commonly found in calcified vessels. Abnormalities in mineral metabolism, particularly hyperphosphataemia and the loss of mineralization inhibitors, initially lead to the formation of calcium phosphate nanocrystals that can transform to more organized and stable apatite crystals. Nanocrystals are taken up by VSMCs via endocytosis and initiate the transdifferentiation into osteoblast-like cells, ultimately resulting in vessel calcification.
Several in vitro and animal studies suggest a protective role of magnesium on vascular calcification by multiple molecular mechanisms (figure 4): Firstly, magnesium inhibits the formation of apatite crystals and forms smaller, more soluble deposits (Peters et al. 2001). Secondly, magnesium functions as a calcium antagonist thus inhibiting the entry of calcium into the cells (Altura et al. 1987). Thirdly magnesium restores the balance between the expression of calcification promoters and inhibitors (Kircelli et al. 2011). In addition, magnesium acts on the CaSR and activation of CaSR by calcimimetics has been shown to inhibit VSMC calcification (Ivanovski et al. 2005).
Clinical studies provided evidence for the protective effect of magnesium on vascular calcification. Mitral annular calcification and increase of carotid intima–media thickness were shown to be strongly associated with low magnesium levels in HD patients (Tzanakis et al. 2004; Tzanakis et al. 1997). Long-term magnesium supplementation reduced carotid intima–media thickness and thus may retard arterial calcification in CKD patients (Turgut et al. 2008). Epidemiological studies in HD patients revealed that elevated magnesium levels are associated with survival advantages, whereas low magnesium levels are independent predictors of death (Ishimura et al. 2007). Overall, elevated serum magnesium levels and/or magnesium supplementation may delay arterial calcification and lead to survival advantages in dialysis patients. However, adequately powered randomized studies are required to confirm these results.
MAGNESIUM AS A PHOSPHATE BINDER
Declining renal function is associated with increasing phosphate levels and most patients will have hyperphosphataemia from CKD stages 4 onwards. Phosphate binders are needed in CKD patients to prevent hyperphosphataemia leading to disturbed bone and mineral metabolism, cardiovascular disease and sHPT. Phosphate binders have been used for many years, but the ideal binder in terms of efficacy, patient adherence, safety and costs has not been found (Hutchison et al. 2012). phosphate binders containing aluminium or calcium have well-known drawbacks, like Aluminium-toxicity or increased calcium load causing hypercalcaemia and associations with vascular calcification. Newer agents avoid calcium burden but are very expensive. Magnesium-containing phosphate binders offer a chance to circumvent some of the problems associated with other agents.
Early studies in the 1980s substituted magnesium hydroxide for aluminium, because of its reported toxicity. Magnesium hydroxide was effective in lowering phosphate and PTH levels, but was associated with poor gastrointestinal tolerability. Later studies used magnesium carbonate combined with calcium carbonate or calcium acetate to improve the tolerability. The results of some of the most important recent studies are described below.
Efficacy of magnesium carbonate in combination with calcium carbonate or calcium acetate
A randomized parallel-group study compared the efficacy of magnesium carbonate/calcium acetate compared to calcium carbonate monotherapy in 50 HD patients (Deuber et al. 2004). Treatment with magnesium carbonate/calcium acetate resulted in significantly lower serum phosphate, calcium and iPTH levels compared to monotherapy (p<0.05 each). Serum magnesium concentrations in the combination group were significantly increased, but remained within the normal range (Deuber et al. 2004).
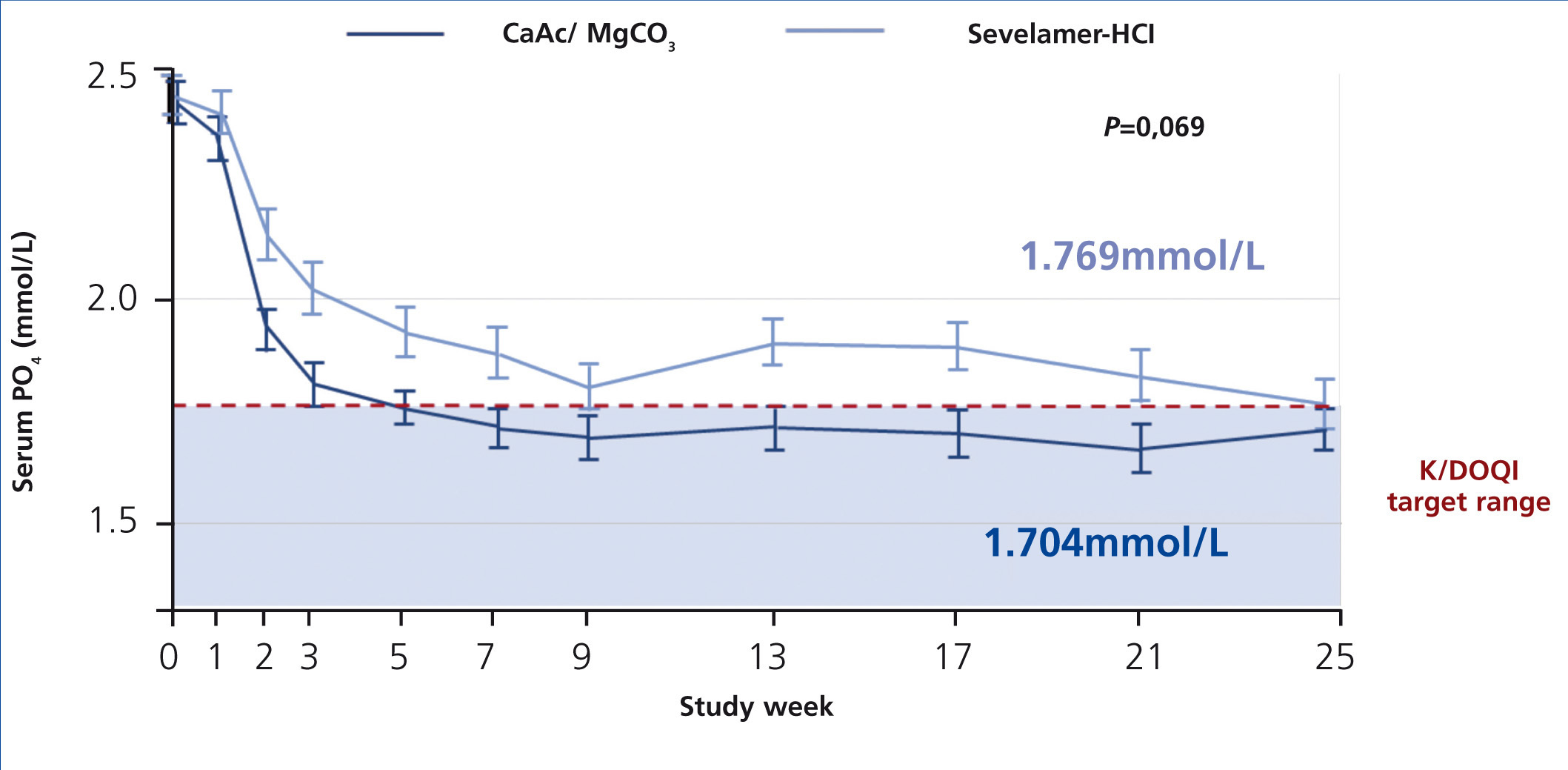
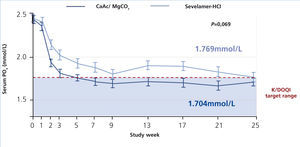
The CALMAG study, a large randomized multicentre study, compared the efficacy of calcium acetate/magnesium carbonate to sevelamer hydrochloride in 255 HD patients (de Francisco et al. 2010). The primary efficacy endpoint was to show non-inferiority of calcium acetate/magnesium carbonate compared to sevelamer hydrochloride in lowering phosphate levels to below K/DOQI targets after 24 weeks of treatment. This was fulfilled, and there was no significant difference between groups regarding the lowering of serum phosphate levels at the end of the study (p=0.069) (figure 5). The area under the curve (AUC) for serum phosphate (p=0.0042) and the number of visits above K/DOQI (≤1.78 mmol/L, p=0.0198) and KDIGO targets (≤1.45 mmol/L, p=0.0067) were significantly lower with calcium acetate/magnesium carbonate compared to sevelamer hydrochloride.
Safety of magnesium carbonate in combination with calcium carbonate or calcium acetate
Tolerability, hypermagnesaemia and calcium load are relevant safety parameters of magnesium-containing phosphate binders. The tolerability was shown by different studies. Combined magnesium/calcium carbonate was compared to calcium carbonate monotherapy in a 2-year, randomized, crossover trial in 29 HD patients (Delmez et al. 1996). Serum levels of calcium, phosphate and magnesium were similar in both phases, but the ingestion of calcium was significantly less in the combined phases (p<0.0001). In spite of the relative high magnesium intake of 465±52 mg/day (mean±SD), the combined treatment was generally well tolerated: No patients reported any gastrointestinal symptoms including loose stools, diarrhoea or bloating.
A pilot study investigated the calcium load of magnesium/calcium carbonate compared to calcium acetate monotherapy (Spiegel et al. 2007). 30 HD patients were randomized to receive either combination (n=20) or monotherapy (n=10) for 12 weeks. Phosphate control of the combination therapy was at least as good as that of calcium acetate alone, but calcium consumption was significantly lower in the magnesium/calcium carbonate group (908±24 versus 1743±37 mg/day, mean±SD; p<0.0001) resulting in significantly lower calcium levels (p<0.003). Both regimens were generally well tolerated with a similar incidence of gastrointestinal symptoms.
The CALMAG study compared safety parameters of calcium acetate/magnesium carbonate to sevelamer hydrochloride (de Francisco et al. 2010). Both regimens were equally well tolerated with a similar number of adverse events. No symptomatic hypermagnesaemia was observed. Although a small increase in serum magnesium occurred in the calcium acetate/magnesium carbonate group, the mean concentration was well below possibly symptomatic levels (1.29 ± 0.25 versus >1.5-2.0 mmol/L).
Spiegel et al. (2009) studied the effects on coronary artery calcification (CAC) and vertebral bone mineral density (V-BMD) of magnesium plus calcium carbonate in a small, 18-month, open-label pilot study with 7 HD patients. They found no significant progression of the CAC score and no significant change in V-BMD, suggesting that magnesium may have a favourable effect on these parameters, though the size of the study precludes any firm conclusions and larger studies are needed to confirm these findings.
Cost-effectiveness
Healthcare costs become an ever more important factor. Magnesium containing phosphate binders are relatively inexpensive compared with sevelamer- or lanthanum-based agents. Calculated costs for the treatment with magnesium carbonate/calcium acetate were about 80% lower compared to sevelamer hydrochloride (de Francisco et al. 2010). Though magnesium-calcium-based salts have proven efficacy and safety, there are still some concerns about hypermagnesaemia and serum magnesium monitoring. Serum magnesium levels are not routinely measured in many dialysis clinics. Some may argue that routine monitoring is not necessary since the safety margin appears significant, but this is unlikely to satisfy the concerns of nephrologists. The cost of introducing magnesium monitoring would, however, be offset by the lower cost of magnesium-containing phosphate binders over sevelamer or lanthanum, and most modern multi-channel biochemistry analyzers can report magnesium if required without major cost implications(as an example: magnesium in serum (photometric assessment/AAS)—Germany (Synlab, Augsburg): GOÄ 3621 1.00 (GOÄ = Gebührenordnung für Ärzte, private; scale of charges for physicians)= 2.33 €; France (Biomnis, Ivry-sur Seine)= 1.89 €).
CONCLUSION
Despite its physiological importance, the clinical role of magnesium in terms of benefits and harms of higher or lower magnesium levels particularly in CKD patients has been underestimated for many years. Disorders of magnesium balance, especially hypomagnesaemia, are common, but hardly identified in routine clinical practice. Furthermore magnesium deficiency was shown to be a risk factor for several common metabolic diseases, whereas magnesium supplementation and mild hypermagnesaemia might have beneficial effects in CKD patients with regard to calcification and mortality. On the other hand, the growing understanding of the effects of hyperphosphataemia has raised the need for efficient and well-tolerated phosphate binders. The combination of calcium acetate and magnesium carbonate presents a phosphate binder, which is at least as effective as sevelamer hydrochloride and equally well tolerated, but saves about 80% of treatment costs. Magnesium levels were only slightly elevated and there was no difference in gastrointestinal tolerability compared to sevelamer hydrochloride. If necessary, routine magnesium monitoring is feasible without major cost implications. The possible beneficial effects of magnesium regarding calcification, PTH-levels and survival in CKD patients on the one hand and the promising results about efficacy, tolerability and cost-effectiveness on the other are potential advantages which make magnesium-containing phosphate binders an attractive treatment option in routine clinical practice. However, more clinical research is needed to confirm and understand the clinical effects of magnesium administration.
Acknowledgments
This work is based on the special issue of Clinical Kidney Journal: Magnesium - a versatile and often overlooked element: New Perspectives with a focus on chronic kidney disease (Vol 5, S1, February 2012). We acknowledge the contributions of the following authors: Friedrich C Luft; Wilhelm Jahnen-Dechent; Markus Ketteler; Jeroen HF de Baaij; Joost GJ Hoenderop; René JM Bindels; Helmut Geiger; Christoph Wanner; John Cunningham; Piergiorgio Messa; Ziad A Massy; Tilman B Drüeke; Alastair J Hutchison; Martin Wilkie.
We would also like to thank Jutta Passlick-Deetjen, Kristina Gundlach, Mirjam Peter, Nora Burkard, and Sonja Steppan for their valuable contributions to the organisation and development of this update.
KEY CONCEPTS:
- Hypomagnesaemia prevalence is high and associated with several diseases, however it is often undetected.
- Moderate hypermagnesaemia seems to have positive effects on vascular calcification and mortality rates.
- In dialysis patients serum magnesium is highly influenced by magnesium dialysate concentration.
- Mg-carbonate/Ca-acetate is an efficient, safe and cost-effective phosphate binder.
Conflict of interest
The authors declare potential conflicts of interest.
Funding: Abbott, Amgen, Fresenius.
Publication fees: Abbott, Amgen, Fresenius, Shire.
Consulting fees: Amgen, Fresenius, Vifor, Shire.
Per diems and travel expenses Abbott, Amgen, Fresenius,
Vifor, Sanofi, Shire.
Table 1. Influence of magnesium dialysate concentrations on serum magnesium levels in HD and PD patients
Figure 1. Regulation of magnesium homeostasis.
Figure 2. Overview: Role of magnesium in different diseases.
Figure 3. Relationship between fractional excretion of magnesium and endogenous creatinine clearance in CKD patients
Figure 4. The putative protective roles of magnesium in the course of vascular calcification.
Figure 5. Efficacy of calcium acetate and magnesium carbonate (CaAc/MgCO3) compared to sevelamer hydrochloride on lowering of serum phosphate levels