Antecedentes y objetivos: Se ha evaluado el uso de darbepoetina alfa en la primera semana postrasplante renal (TR). Métodos: Estudio observacional, retrospectivo, realizado en cuatro hospitales, que incluyó todos los pacientes adultos que fueron sometidos a TR durante un período de 9 meses con los datos de hemoglobina (Hb) durante los primeros 6 meses postrasplante (n = 129). Darbepoetina alfa fue administrada de acuerdo con la práctica clínica. Resultados: Se administró darbepoetina alfa en la primera semana a 60 individuos (46,5 %), los cuales presentaban una Hb media (± desviación estándar) basal de 12,7 ± 1,6 g/dl. La incidencia de anemia (Hb < 11 g/dl) durante el primer mes fue superior en los pacientes que no recibieron darbepoetina alfa (40,6 % vs. 25,0 % en los pacientes tratados, p = 0,045). No se observaron diferencias en la incidencia de la anemia entre los meses +2 a +6. Hubo una tendencia hacia la disminución de transfusiones en los pacientes que recibieron darbepoetina alfa (13,3 % vs. 20,3 %, p = 0,295). El tiempo de recuperación renal fue similar, pero, en el subgrupo que recibió injertos procedentes de donantes en asistolia, hubo una tendencia hacia una recuperación más rápida con darbepoetina alfa (15,1 ± 7,7 vs. 20,1 ± 8,8 días, p = 0,157). El aclaramiento de creatinina a los 3 y 6 meses fue similar. Catorce pacientes (10,9 %) sufrieron un evento cardiovascular, sin relación con darbepoetina alfa (p = 0,772). Conclusiones: La administración de la darbepoetina alfa en la primera semana tras el trasplante renal reduce la incidencia de anemia durante el primer mes, sin aumentar los eventos cardiovasculares.
Background and objectives: The use of darbepoetin alfa in the first week of post-renal transplant (RT). Methods: Retrospective observational study carried out in four hospitals, which included all adult patients that underwent RT for 9 months with haemoglobin data during the first 6 months of post-transplant (n=129). Darbepoetin alfa was administered in accordance with the clinical practice. Results: Darbepoetin alfa was administered in the first week to 60 individuals (46.5%), who had a mean baseline Hb (±standard deviation) of 12.7g/dl±1.6g/dl. Anaemia incidence (Hb<11g/dl) during the first month was higher in patients who did not receive darbepoetin alfa (40.6% vs. 25.0% in patients treated with darbepoetin alfa, P=.045). No anaemia incidence differences were observed during months +2 to +6. There was a tendency towards transfusion decrease in patients who received darbepoetin alfa (13.3% vs. 20.3%, P=.295). Renal recovery time was similar but in the subgroup which received grafts from donors with asystole there was a tendency towards a faster recovery with darbepoetin alfa (15.1±7.7 vs. 20.1±8.8 days, P=.157). The creatinine clearance rate at 3 and 6 months was similar. Fourteen patients (10.9%) suffered from cardiovascular events with no relation to darbepoetin alfa (P=.772). Conclusions: Administering darbepoetin alfa in the first week following renal transplant reduces anaemia incidence during the first month without increase cardiovascular events.
INTRODUCTION
Post-transplant anaemia (PTA) is a common complication during the first few months following transplantation. The TRansplant European Survey on Anaemia Management (TRESAM) reported a 38.6% prevalence rate of anaemia among transplant recipients during the first 5 years post-transplant,1 similar to the rates observed in other studies.2 The aetiology of PTA is not well defined. The primary factors associated with this entity are low serum levels of erythropoietin, renal failure, and iron deficits.3 The graft does not start to synthesise erythropoietin until adequate renal function is reached.4-6 This delay can produce anaemia, requiring red blood cell transfusions. In addition, erythropoietin deficits are associated with delayed cellular recovery following episodes of acute tubular necrosis.7,8
The long-term consequences of PTA and delayed graft function on the evolution of the kidney transplant can have major impacts on renal transplant recipients. Anaemia has been associated with left ventricular hypertrophy in patients on dialysis,9 and constitutes an independent predictor for cardiovascular mortality in patients with renal transplants.10 The number of patients on the waiting list for donated kidneys continues to increase, and many of these individuals have poor cardiovascular risk profiles that reduce their tolerance to low haemoglobin (Hb) levels. In addition, the recent increases in the use of expanded-criteria donors for renal transplants increases the probability of delayed allograft function and a slower recovery of renal function. A recent meta-analysis demonstrated that delayed allograft function is associated with a lower 3-year graft survival.11
Currently, no clearly established treatment regimens exist for managing PTA. One issue that remains to be solved is whether patients should receive erythropoiesis-stimulating agents (ESA) during the immediate post-transplant period. The administration of recombinant human erythropoietin (rHuEPO) in kidney recipients during the first few weeks post-transplant has not been shown to provide benefits in terms of correcting immediate postoperative haematocrit values, despite relative resistance to ESA.12 Controversy also exists regarding the need to provide iron supplements as a concomitant treatment along with ESA. Patients with recent transplants have elevated ferritin levels and/or elevated transferrin saturation index (TSAT). Some doctors have interpreted these findings as acute phase reactions to postoperative inflammation, which would imply that there is no need for administering iron supplements. Also, heavy use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB) in kidney transplant recipients can induce resistance to ESA, since both drugs inhibit erythropoiesis.
Few studies have evaluated the efficacy of darbepoetin alfa for treating anaemia in kidney transplant recipients,13-16 and only one of these examined this type of treatment in the immediate postoperative period.16 The primary objective of our observational, retrospective study was to describe recovery from post-transplant anaemia based on the use of darbepoetin alfa during the first week post-transplant.
METHOD
We performed a multi-centre, observational, retrospective study at 4 Spanish hospitals.
Patients
We reviewed the clinical histories of all patients who received kidney transplants between April and December 2004, and included all individuals older than 18 years of age for which Hb levels were available for the first 6 months post-transplant. Patients were excluded if they had an iron deficit (defined as serum ferritin <100μg/l and TSAT<20%) during the period of observation (first 6 months post-transplant). Darbepoetin alfa was administered at each centre in accordance with standard clinical practice. Patients were divided retrospectively into two groups based on the use of darbepoetin alfa in the first week post-transplant.
Variables
We compiled information on the following variables: sociodemographic variables, transplant variables, post-transplant data (delayed graft function, defined as a need for dialysis during the first week post-transplant, graft loss, episodes of rejection, and treatments administered including: darbepoetin alfa, ACE inhibitors, ARBs, diuretics, iron, and immunosuppressants), and laboratory parameters (according to the standard practice at each hospital, determined approximately at the start of the study [time of transplant] and after 1, 2, 3, and 4 weeks and 2, 3, and 6 months).
Evaluation criteria
The primary criterion evaluated was the percentage of patients with Hb<11g/dl during the first month (at least 2 values below 11g/dl in weeks 3 or 4). The secondary variables studies were: percentage of patients with Hb<11g/dl (at least one value below 11g/dl) during the period of 2+ to 6 months post-transplant, number of transfusions required during the first 6 months post-transplant, time to recovery of renal function (defined as a decrease in serum creatinine of at least 0.1mg/dl in comparison with the first post-transplant value and no later increase in the absence of dialysis treatment), glomerular filtration rate (GFR) at 3 and 6 months post-transplant (calculated using the Cockcroft-Gault equation17), and percentage of patients with cardiovascular events.
Statistical analysis
We calculated summary statistics for continuous and categorical variables. Analyses were based solely on observed data. The differences between groups for qualitative variables were evaluated using chi-square or Fisher’s exact tests, as appropriate. Differences in mean levels between groups and compared to baseline levels were evaluated using Student’s t-tests and paired t-tests, respectively. All tests were bilateral, with a 5% significance level. All statistical analyses were performed using SPSS® statistical software, version 13.0 (SPSS Inc., Chicago, Illinois, USA).
Ethical considerations
Our study protocol was approved by the Ethics Committees at all participating hospitals, in accordance with legislation on retrospective observational studies. All information that could identify individual patients was dissociated from our data in order to maintain confidentiality.
RESULTS
Characteristics of the study sample and treatments administered
Of the 162 adult patients who received kidney transplants at the participating hospitals between April and December 2004, 129 complied with the selection criteria and were included in our study (33 cases were excluded, 2 due to a lack of follow-up of Hb levels and 31 due to low ferritin levels, rates, or transferrin saturation [TSAT]). We did not observe significant differences between included and excluded patients (data not shown). Darbepoetin alfa was administered (subcutaneously) to 60 individuals (46.5%) during the first week post-transplant. Of these, 19 patients only received this drug for the first week, 15 during one month, 7 during 3 months, and 19 during the entire 6-month study period. Thirty-three patients that did not receive treatment during the first week then received darbepoetin alfa at some point between 2 weeks and 6 months (of which 12 started treatment between months 1 and 2 and continued on darbepoetin alfa until month 6). Figure 1 displays the distribution of the study population based on treatment with darbepoetin alfa.
The mean dose (± standard deviation) during the first week was 56.0±21.3μg (range: 20-120μg), and all patients received treatment once per week. At 6 months, 34.6% of patients treated with darbepoetin alfa (n=27) had switched to a regimen receiving treatment every two weeks, and 7.7% had switched to a monthly schedule.
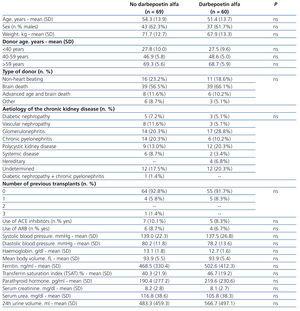
Table 1 summarises patient characteristics and laboratory parameters at baseline measurements based on the use of darbepoetin alfa in the first week post-transplant. We did not observe significant differences for any of the variables measured, not even pre-transplant Hb (13.1±1.8g/dl vs. 12.7±1.6g/dl for untreated and treated patients, respectively; P=.187).
A small percentage of patients were treated with ACE inhibitors at some point during the study period (9.3% at the start of the study, 7.0% at 2 weeks, 7.0% at one month, 11.6% at 3 months, and 14.0% at 6 months), and enalapril was the most commonly used drug. ARBs were administered in 7.8% of patients at the start of the study, in 2.3% at 2 weeks, 6.2% at 1 month, 16.3% at 3 months, and 20.9% at 6 months. Few patients received diuretics (10.1% at the start of the study, 17.8% at 2 weeks, 13.2% at 1 month, 12.4% at 3 months, and 14.7% at 6 months).
At the start of the study, 18.6% of patients received iron supplements, but only one patient continued on this treatment until week 3. At 2 months, three patients started iron supplement therapy, and at 6 months, only two patients were receiving iron.
As regards immunosuppressant treatment, 49.6% of patients received induction therapy with antibodies (OKT3 [n=5], thymoglobulin [n=1], or anti-CD25 antibodies [n=58]). During the study, all patients were treated with a triple regimen involving a calcineurin inhibitor (81.6% tacrolimus and 18.4% cyclosporine), an anti-metabolite (99.1% mycophenolate mofetil and 0.9% enteric-coated mycophenolic acid), and corticosteroids.
Incidence of anaemia and transfusion requirements
In the overall study sample, 12.7% of patients had anaemia (Hb<11g/dl) at the start of the study, and this percentage increased to 74.8% at the end of one week post-transplant. The prevalence of anaemia at 6 months was 14.5%.
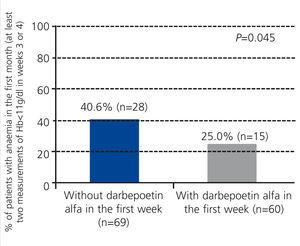
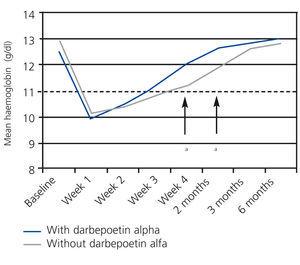
The percentage of patients with anaemia (Hb<11g/dl) during the first month was significantly higher in the group of patients that did not receive darbepoetin alfa (40.6% vs. 25.0% in treated patients; P=.045; Figure 2). We did not observe differences in the period of +2 to +6 months post-transplant (data not shown). Figure 3 displays the evolution of mean Hb levels based on the use of darbepoetin alfa. We observed statistically significant differences between the two groups in week 4 (mean 12.0g/dl in treated patients vs. 11.2g/dl in untreated patients; P=.016) and at 2 months (12.7g/dl in treated patients vs. 11.9g/dl in untreated patients; P=.016), reflecting a faster recovery from anaemia in patients treated with darbepoetin alfa during the first week post-transplant.
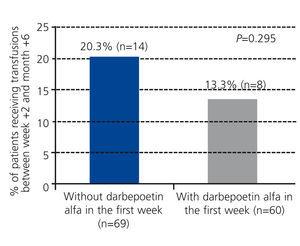
The group of patients treated with darbepoetin alfa required fewer transfusions in the 6 month period following transplantation (13.3% of patients with at least one transfusion, mean number of transfusions: 0.77±1.11) as compared to untreated patients (20.3% with at least one transfusion, mean number of transfusions: 0.85±1.28), although this difference was not statistically significant (P=.295; Figure 4).
Renal function
In the global sample, 43.4% of patients developed delayed graft function. The mean time to recovery of renal function was 12.1±7.4 days (median: 8 days; range: 3-34 days). We did not observe statistically significant differences between patients treated with darbepoetin alfa and untreated patients: 45% of treated patients had delayed graft function as compared to 42% of untreated patients (P=.734).
In the group of patients that received organs from non-heart beating donors (n=24, there were no cases of marginal donors), there was a tendency towards a shorter time to recovery of renal function in patients treated with darbepoetin alfa during the first week (15.1±7.7 days vs. 20.1±8.8 days for untreated patients, P=.157), both for patients with anaemia (17.3±4.0 days vs. 20.2±9.3 days; P=.623) and non-anaemic patients (14.1±8.9 days vs. 19.5±7.8 days; P=.471). We did not observe any differences in the use of darbepoetin alfa in patients who received organs from brain-dead donors (n=66) (data not shown).
Regardless of the use of darbepoetin alfa, we observed a tendency towards a faster recovery of renal function in non-anaemic patients at 1 month, both in patients who received organs from non-heart beating donors and from brain dead donors: 15.3±8.5 days in non-anaemic patients vs. 19.6±8.5 days in anaemic patients (P=.246), and 9.2±4.0 days vs. 12.5±7.9 days in anaemic patients (P=.080), respectively.
GFR at 3 and 6 months post-transplant was similar in patients with and without darbepoetin alfa treatment during the first week: 52.0±24.3ml/min and 51.9±18.7ml/min, respectively, at 3 months, and 52.0±22.9ml/min and 51.9±17.5ml/min at 6 months.
Patient and graft survival and episodes of acute rejection
Only one death was registered (0.8%), which occurred at 6 months in a patient that did not receive darbepoetin alfa, due to sepsis following an episode of acute rejection. There were 8 cases of early graft loss due to surgical complications (3 in the group receiving darbepoetin alfa and 5 untreated patients). Another 3 patients suffered late graft loss: one at week 4 due to cortical necrosis (in a patient who received darbepoetin alfa), one at month 2 due to an infection of the surgical wound and a urinary fistula (in a patient without darbepoetin alfa), and one at month 3 of an unspecified cause (in a patient who received darbepoetin alfa). There were also 42 episodes of acute rejection registered in 36 patients (27.9%), of which 23 had received darbepoetin alfa during the first week post-transplant. We did not observe significant differences in terms of the incidence of acute rejection between patients with and without darbepoetin alfa treatment (21.7% vs. 33.3%, respectively; P=.141).
Cardiovascular events
Fourteen patients (10.9%) suffered a cardiovascular event during the first 6 months post-transplant (9 arrhythmias [4 in patients with darbepoetin alfa and 5 in untreated patients], 2 cerebrovascular accidents (one in week 1 in an untreated patient and one in month 2 in a patient with darbepoetin alfa], one myocardial infarction at the start of the study, in an untreated patient, one transitory ischaemic attack at 6 months, in an untreated patient, and one case of angina with arrhythmia [in week 1, in a patient on darbepoetin alfa]). We did not observe any correlation between the occurrence of cardiovascular events and the administration of darbepoetin alfa (8 events in patients without darbepoetin alfa and 6 in patients with darbepoetin alfa; P=.772).
DISCUSSION
In this observational study, darbepoetin alfa was administered to almost half of all patients (46.5%) during the first week post-transplant, which was associated with a lower incidence of anaemia at the end of the first month. The use of ESA during the first weeks following transplantation varies widely among different hospitals. The results from the TRESAM study revealed that, of the 8.5% of patients with severe anaemia, only 17.8% were treated with erythropoietin.1 Curiously, the use of darbepoetin alfa in our study was not associated with the presence of pre-transplant anaemia. We did not observe significant differences in any other of the variables examined (including donor characteristics and iron parameters) between patients treated with darbepoetin alfa and untreated patients, making it difficult to determine what factors influenced the decision to prescribe this medication. Despite this limitation, our results demonstrate that the use of darbepoetin alfa in the immediate post-transplant period reduces the risk of anaemia during the first month by 50%, with no clinically relevant adverse side effects.
In a previous randomised clinical trial with rHuEPO,18 in which 40 patients with kidney transplants were randomised to receive 100IU/kg of rHuEPO three times per week if their Hb levels decreased below 12.5g/dl as compared to a control group, the time to reach an Hb value >12.5g/dl was shorter in the rHuEPO group, although no statistically significant differences were observed in terms of the proportion of patients who reached a value >12.5g/dl. The authors concluded that the use of rHuEPO, at doses equivalent to those used in our study for darbepoetin alfa, provides limited benefits for correcting anaemia. The primary difference between the two studies was the initial value of Hb, which was much higher in our study. Baseline serum levels of ferritin and creatinine were also considerably higher in our study, which indicates a worse initial state of the kidney graft. Another study16 also failed to demonstrate a significant benefit from administering darbepoetin alfa during the first months post-transplant. It is possible that the benefits from ESA during the immediate post-transplant period depend on the initial situation of the recipient. Nampoory et al.19 analysed endogenous erythropoietin levels (EPO) in both anaemic and non-anaemic stable kidney recipients, observing that non-anaemic recipients had normal or abnormally high serum EPO levels (“EPO resistance”), whereas almost all patients with anaemia had EPO deficits and responded rapidly to low doses of rHuEPO.
The prevalence of anaemia in our study sample was quite high. It is remarkable that, in the first week post-transplant, Hb levels had descended below 11g/dl in the vast majority of patients (74.8%). This value is higher than that reported in a similar observational study carried out in France (cumulative prevalence of anaemia: 66%).20 The elevated baseline levels of creatinine and the use of mycophenolate mofetil in almost all patients could help explain the high rate of anaemia.21
In the group treated with darbepoetin alfa, 25% of patients were anaemic at the end of the first month, which suggests the presence of unresponsive individuals in this sample. This finding could be related to the state of acute inflammation in these patients,22 as reflected by their elevated ferritin levels. A recent study compared gene expression in kidney recipients treated with ESA to gene expression in non-anaemic controls, and demonstrated that anaemic patients exhibit an over-expression of molecules implicated in the immune response, which suggests that the inflammation cascade is involved in the development of anaemia following transplantation.23 Other explanations could involve the use of ACE inhibitors and ARBs in approximately 15% of patients.24 The prevalence of anaemia at 1 month in the untreated group (40.6%) is similar to values reported in the TRESAM study (38.6%), although this value decreased considerably after 6 months (14.5% of the entire sample).
We observed a tendency towards a reduced requirement for transfusions in patients treated with darbepoetin alfa, although this difference did not reach statistical significance, perhaps due to the small number of patients with severe anaemia (requiring transfusions) at the start of treatment. The doses administered (56μg/week) were higher than those recommended by clinical guidelines for the correction phase (31.5-42mg/week for a patient weighing 70kg),25 but are comparable to those reported in similar observational studies.20
As regards the relationship between the use of darbepoetin alfa and recovery of renal function, we did not observe a significant influence in our study sample. However, when considering only those patients who received organs from non-heart beating donors, we observed a tendency towards a shorter time needed to recover renal function in patients treated with darbepoetin alfa, both in anaemic and non-anaemic patients. Despite the small sample size in this group, our findings are in line with those from studies that have shown independent effects of ESA on a faster recovery from acute tubular necrosis.7,8 Results from in vitro animal studies suggest that administering erythropoietin before or during the ischaemic damage caused to renal epithelial tubular cells protects these cells against cell death through the JAK2/Y343/STAT5 signalling pathway.26
Our results also suggest that the presence of anaemia at 1 month is associated with delayed graft function. This relationship has also been previously reported,27 and could be explained by the causal effect of renal failure on anaemia.12
The use of darbepoetin alfa in our sample did not produce an increase in the incidence of cardiovascular events, and renal function after 3 and 6 months was similar between treated and untreated patients. Patient and graft survival rates were high. In the TREAT study (The Trial to Reduce Cardiovascular Events with Aranesp Therapy), the risk of cerebrovascular accidents increased in pre-dialytic patients with diabetes treated ESA in order to reach a target Hb of 13g/dl.28 In order to prevent over-dosing and unnecessary adverse side effects from treatment with ESA, these parameters must be strictly monitored during the first months following transplantation, with frequent clinical and biochemical evaluations so as to suspend treatment as soon as abnormal values are detected.
The primary limitations to our study are related to its observational and retrospective design, the heterogeneity in clinical practice protocols due to the inclusion of multiple hospitals, the lack of information regarding the motives for prescribing darbepoetin alfa and the doses used, and the small sample size. As such, ours was an exploratory study, and its results must be interpreted with this in mind.
In conclusion, the administration of mid-range doses of darbepoetin alfa in the immediate post-transplant period contributes to the normalisation of Hb levels towards the end of the first month in kidney transplant recipients, and could be related to a faster recovery of renal function in patients at a risk for delayed allograft function, with no clinically relevant adverse side effects. Given the greater rates of mortality and morbidity observed in kidney transplant recipients with anaemia,29-32 the use of ESA can be considered during the first few weeks following transplantation. More studies will be needed to investigate the factors that serve as predictors for the response to this type of treatment, so as to improve the cost-effectiveness of ESA therapy, perhaps based on endogenous levels of EPO or other parameters of renal function.
Authorship and conflicts of interest
CJ, MM, AA, and JP designed the study and coordinated the group; CJ participated in data analysis and manuscript elaboration; all other authors contributed to clinical data collection and manuscript revision. This study was financed by Amgen SA. Amgen SA also provided financial support for scientific writing. The authors declare that they have no conflicts of interest related to the contents of this article.
Declarations
Source of funding (in the form of grants, equipment, medications, or all)
This study was financed by Amgen SA. Amgen SA also provided financial support for the scientific writing process. The authors declare that they have no other conflicts of interest.
Acknowledgements
This study was financed in part by a grant from Amgen, SA. Neus Valveny, of Trial Form Support, provided assistance in writing the manuscript.
Table 1. Baseline characteristics of study patients based on the use of darbepoetin alfa in the first week post-kidney transplant
Figure 1. Administration of darbepoetin alfa in the study population during the first 6 months post-transplant
Figure 2. Percentage of patients with anaemia in month 1 based on the use of darbepoetin alfa in the first week
Figure 3. Evolution of mean haemoglobin levels between baseline measurement and six months based on the use of darbepoetin alfa in the first week
Figure 4. Percentage of patients with transfusions between week +2 and month +6 based on the use of darbepoetin alfa during the first week