Antecedentes: Ningún estudio ha podido determinar cuál es la mejor ecuación para calcular la función renal en pacientes con lupus eritematoso sistémico (LES) partiendo del análisis de una evaluación de referencia. Objetivo: Evaluar el rendimiento de las ecuaciones basadas en cistatina/creatinina en la estimación de la función renal en pacientes con LES. Métodos: Realizamos el estudio en dos fases: la primera incluía 14 pacientes en los que el aclaramiento de yotalamato se utilizó para determinar la tasa de filtración glomerular (FG) y se comparó con diferentes ecuaciones basadas en cistatina C y/o creatinina En la segunda fase, utilizamos la mejor ecuación (basada en cistatina y creatinina) como «estándar de referencia» para comparar 5 ecuaciones basadas en creatinina en 55 pacientes con LES. Resultados: En la primera fase, la ecuación desarrollada por Stevens et al. (basada en creatinina y cistatina C) fue considerada la mejor. En la fase dos, la ecuación CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) fue considerada la mejor con una desviación de –2,1 ml/min/1,73, exactitud (P30 %) del 94,5 % y precisión (rango intercuartílico de las diferencias ) de –2,1 ml/min/1,73. Conclusiones: Nuestros datos sugieren que la ecuación CKD-EPI es la mejor ecuación basada en creatinina para estimar la FG en pacientes con LES.
Background: No study has determined the best equation for estimating renal function in patients with systemic lupus erythematosus (SLE), starting from a gold standard test. Objective: To evaluate the performance of cistatin/creatinine based equations for estimating renal function in patients with SLE. Methods: We conducted two phases: the first phase included 14 patients in which iothalamate clearance was used to determine the glomerular filtration rate (GFR) and compared with different equations based on cystatin C and/or creatinine. In the second phase, we used the best equation (a cystatin and creatinine-based equation) as “reference standard” to compare 5 creatinine-based equations in 55 patients with SLE. Results: In the first phase the equation developed by Stevens and colleagues (based on creatinine and cystatin C), was the best equation. In phase 2, the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation was the best equation with bias of -2.1ml/min/1.73, accuracy (P30) of 94.5% and precision (interquartile range of differences) of -2.1 ml/min/1.73. Conclusions: Our data suggest that CKD-EPI is the best creatinine-based equation to estimate GFR in patients with SLE.
INTRODUCTION
Renal damage is an important factor of both morbidity and mortality in patients with lupus erythematosus (SLE).1 Determination of renal function has an important bearing on the clinical management, risk stratification and medication dosage adjustment.2
Measurement of urine clearance markers (like inulin) are the gold standard to calculate glomerular filtration rate (GFR); these methods are complex, expensive and cumbersome to perform;3,4 the GFR predictive creatinine-based equations offer a rapid method for assessing kidney function by reliance on serum creatinine and anthropometric data; these equations include: Cockcroft-Gault equation (CG), the Modification of Diet in Renal Disease study (MDRD) equation, the Mayo Clinic Quadratic (MCQ) equation and the CKD-EPI equation.5-7 Creatinine clearance (CrCl) is one other method, commonly used by rheumatologists.8
Performance of creatinine-based equations is known to vary among populations. For example, the MDRD study equation performs well in patients with chronic kidney disease, but is less accurate in potential kidney donors, young people with type-1 diabetes, and patients with substantially reduced muscle mass;9-11 moreover, CG is an estimate of CrCl originally developed in a predominantly male, Caucasian population.12 Conclusions of these and other studies about equations may not be appropriate in SLE, a predominantly female disease,13 encompassing many ethnicities,13 reduced muscle mass,14 and with a wide range of renal function.15 However, there is a paucity of data regarding the equations for estimating renal function in patients with SLE.15-17 Moreover, almost half of the rheumatologists use CrCl in all of their patients in order to estimate GFR, a method with a high rate of inappropriate collection and more expensive than the creatinine based equations.18
To our knowledge, no study has determined the best equation for estimating renal function in patients with SLE, starting from iothalamate urinary clearance as gold standard. Therefore, we performed this study to evaluate which is the best equation, based on serum creatinine, to assess renal function in patients with SLE.
PATIENTS AND METHODS
We included, in consecutive selection, all patients with SLE according to the American College of Rheumatology (ACR) criteria.19,20
Exclusion criteria: patients under 18 years old, asthma, hypothyroidism, current smoking, malignancy, prednisone doses ≥30mg/d (or the equivalent dose of another glucocorticoid), pregnancy and severe disease activity (MEX-SLEDAI≥8).21 All patients signed informed consent and our study was approved by the Central Hospital Institutional Review Board.
Ideal body weight (IBW) was calculated (Robinson equation).22 Body surface index (BSI) was calculated in accordance with the Dubois equation.23
We divided this study into 2 phases.
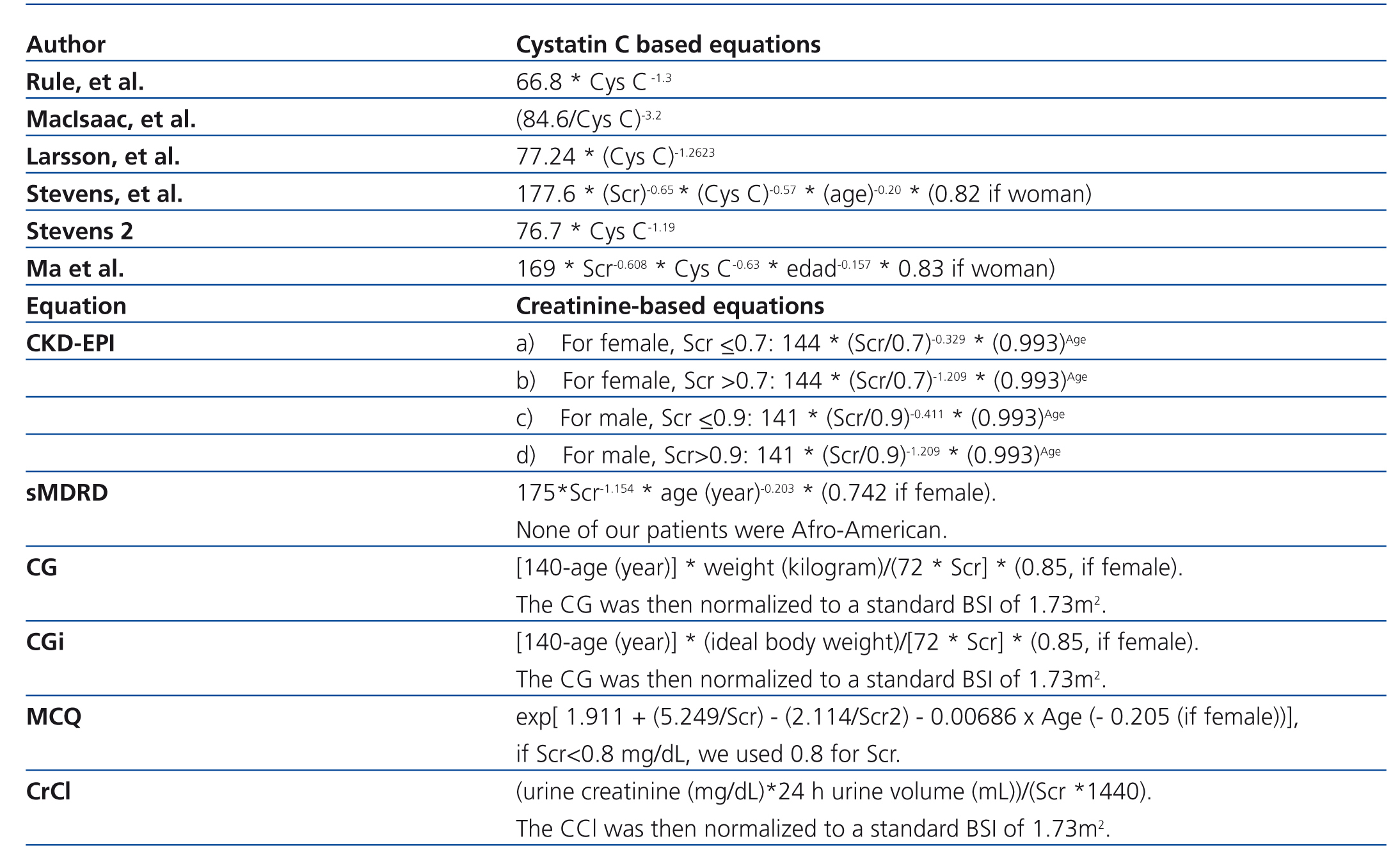
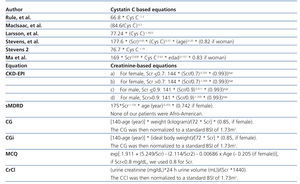
Phase 1: We measured GFR with iothalamate urinary clearance in 14 patients with SLE. GFR calculated through iothalamate was compared with the GFR estimated through cystatin and/or creatinine based equations. The main objective in this phase was to validate the best cystatin/creatinine-based equation to use at the second phase as our reference standard (Table 1).11,24-27 We selected cystatin-based equations for this study from equations developed in other studies taking into account: studies were developed from adult patients, with more than 100 patients, and the measurement of cystatin C had been made by the same method used in our study (immunonephelometry, PENIA).
Phase 2: The best cystatin/creatinine-based equation found in phase I was taken as the reference standard (measured GFR) to assess CrCl and creatinine-based equations (Table 1) in 55 patients (estimated GFR).5,7,28
Laboratory tests
We recalibrated serum creatinine values to standardized creatinine measurements by using the Roche enzymatic method as described elsewhere.10,29
Serum cystatin C was measured using a particle-enhanced immunonephelometric assay (N Latex Cystatin C, Dade Behring, IL). The between-day assay coefficient of variation was 3.5%.
In phase 1, all patients had a non-radiolabeled iothalamate clearance using a previously described standard laboratory method.30 In brief, after oral hydration with 4–6 glasses of water, patients received a subcutaneous injection of non-radiolabeled iothalamate (Conray®). Following a 1-h equilibrium period, the patient voided, the first serum sample was drawn and a timed urine collection was begun. A sonographic scanner assessed bladder emptying and a bladder catheter was placed in patients with urinary retention. Following the timed urine collection (approximately 45–60min), a second serum sample was obtained. GFR was calculated by the clearance equation (UIoV/Pio where UIo and PIo are the urine and plasma concentrations of iothalamate, and V is the urine flow) using the mean of two serum samples and one urine sample assayed for iothalamate via capillary electrophoresis. All GFR measurements were standardized for BSI (per 1.73m2) by multiplying by 1.73 and dividing by BSI.
Statistical analysis
We used R, version 2 (Free Software Foundation, Boston, Massachusetts) to compute all analyses.
Categorical variables are presented as frequencies and percentages, and continuous variables as means and standard deviations, or medians and (minimum-maximum) for variables with skewed distributions.
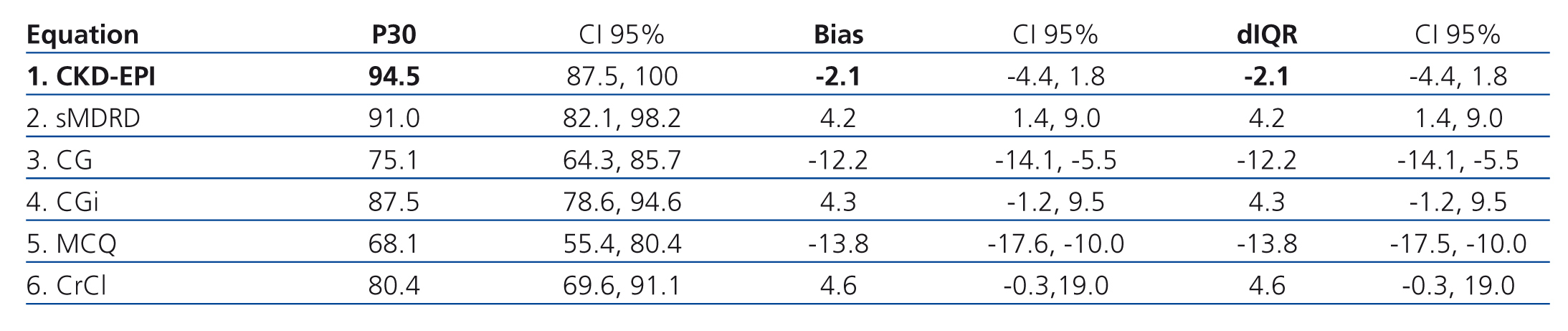
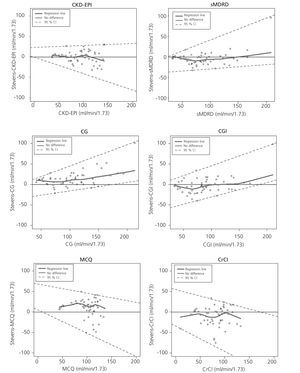
Measured GFR (mGFR) and estimated GFR (eGFR) were compared for each patient graphically by plotting mGFR and the difference (mGFR - eGFR) against eGFR. A smoothed regression line is shown with the 95% confidence interval (95% CI) computed by using the lowest smoothing function in R, and using quantile regression, excluding the lowest and highest 2.5% of estimated GFR. Bias was expressed as the median difference (mGFR - eGFR), with positive values indicating an under-estimation of mGFR. Precision was expressed as interquartile range (IQR) for the differences (dIQR). Accuracy was expressed as the percentage of eGFR within 30% of mGFR (P30). Confidence intervals (CI) were computed by using bootstrap methods (2,000 bootstraps) for median differences and IQR of the differences and by the binomial method for P30.
The reference method or mGFR was iothalamate for phase I, and the best cystatin/creatinine-based equation (Stevens’ equation) for phase 2.
The best equation in both phases was that which had less bias, less dIQR and higher P30.
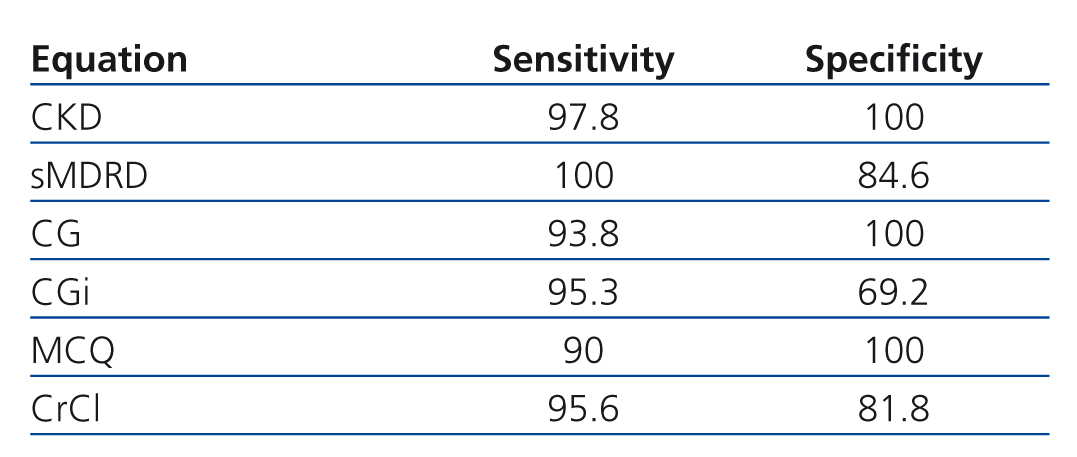
In phase 2 we constructed a 2x2 contingency table to determine the sensitivity and specificity of each equation to classify if each one of the patients had or not had GFR<60 ml/min per 1.73m2.
RESULTS
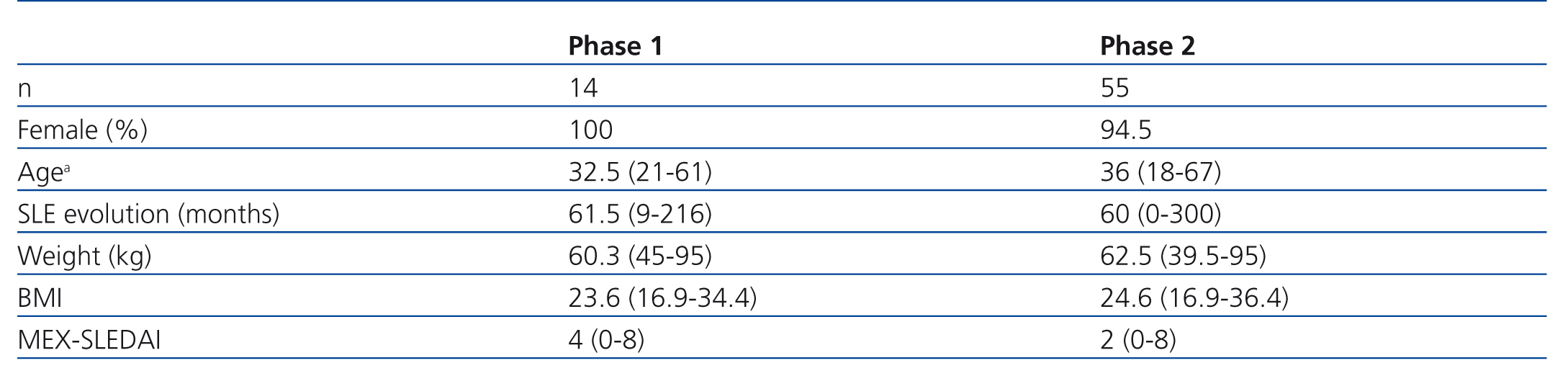
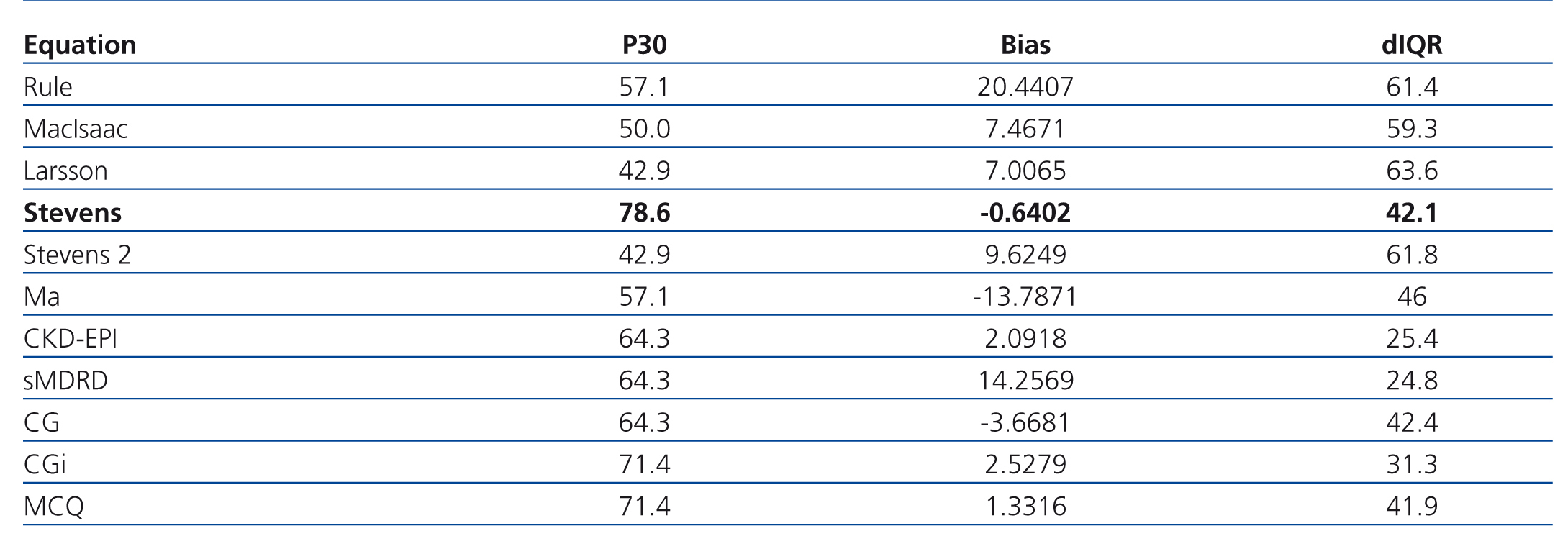
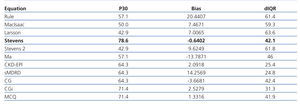
In the first phase we included 14 patients; their median age was 32.5 years old, and 61.5 months of mean SLE evolution time (Table 2). Table 3 shows the results of the first phase which highlights the fact that the equation based on creatinine and cystatin C developed by Stevens and colleagues11 (Stevens’ equation) had the best bias and the most accuracy.
Because our patients were selected for their low disease activity and drug doses, and because of the interference of these factors may have on cystatin C levels, for the clinician, the better setting is to have a creatinine-based equation than a cystatin-based equation, so we performed a second phase with more patients to determine the best creatinine-based equation to estimate GFR in patients with SLE (Table 1).
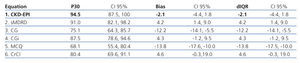
Table 4 shows how the bias, precision and accuracy are better with the CKD-EPI equation than with the others. In this phase, 55 patients were included; median age was 36 years old and SLE evolution time was 60 months (Table 2).
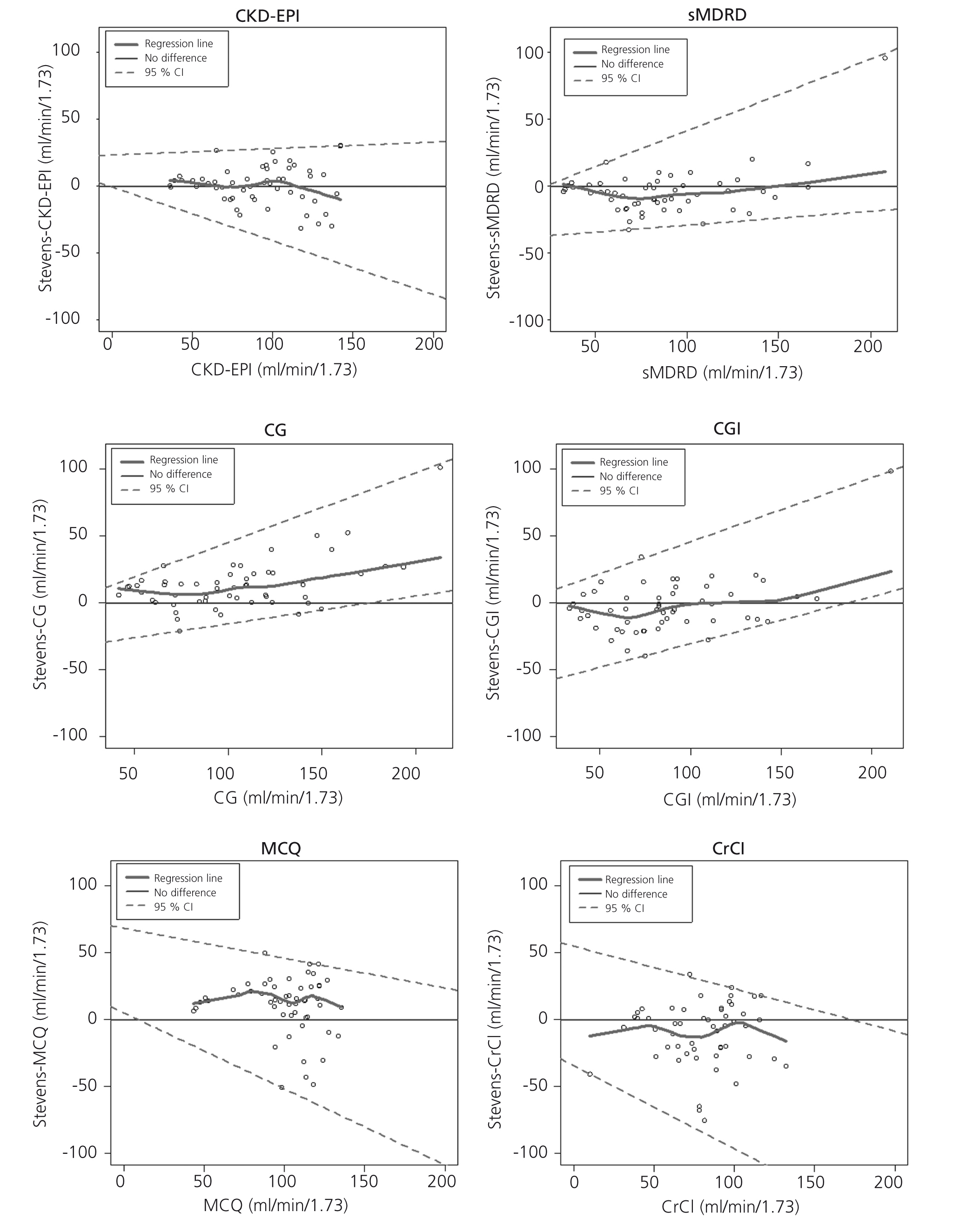
Figure 1 shows the difference between measured and estimated versus estimated GFR in phase 2. A smoothed regression line is shown with the 95% CI, using quantile regression. Notice how the CKD-EPI equation has a good performance both at high and low GFR.
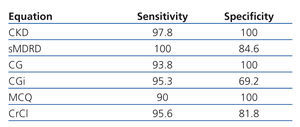
Table 5 shows sensitivity and specificity of each equations to detect GFR less than 60ml/min/1.73. Eleven patients had GFR less than 60ml/min/1.73 according to Stevens’ equation, CKD-EPI misclassified 1 patient (1.8%), sMDRD 2 (3.6%), CG 4 (7.3%), CGi 6 (10.9%), MCQ 5 (9.1) and CrCl 4 (7.3%).
DISCUSSION
The GFR should be determined as accurately as possible, ideally with an accessible and inexpensive method which does not cause harm to the patient. Several factors affect serum creatinine level other than GFR, including its generation from muscle metabolism; these factors compromise the generalization of the equations in patients with SLE. Cystatin-based equations has no dependence on muscle mass, but previous studies showed evidence for non-GFR determinants of cystatin C (inflammation, glucocorticoids, thyroid disease and cytotoxic drugs); variation in these factors can restrict the use of this protein for measuring GFR in patients with SLE.31 Even though we reported previously that Cystatin C does not correlate with disease activity and drug doses,32 we selected our patients avoiding these factors.
Because there are multiple equations to estimate GFR, the management guidelines of patients with SLE are based on studies in other different diseases from SLE and we think that the results on these diseases cannot be extrapolated to SLE.8 National Kidney Foundation (NKF) guidelines suggest using the GFR estimating equations (not the CrCl) to achieve a better assessment of renal function.6,7 In spite of these recommendations, 24 h urine collection to determine CrCl is a widely used method for assessing GRF in patients with SLE (including clinical trials).16,33-35
Our study identifies several important findings: 1) The equation developed by Stevens and colleagues based on creatinine and cystatin C is the best equation to estimate renal function for our specifically selected SLE patients (with low disease activity, low doses of glucocorticoids, no smoking, no hypothyroidism). 2) Because of all those factors that the clinicians must take into account when using cystatin C in a patient with SLE (smoking, hypothyroidism, etc.), we looked for an equation based only on serum creatinine in which there is not the influence of these factors and our results were: the CKD-EPI equation should be used in patients with SLE.
The importance of our results is that we do not need a 24h urine collection to evaluate renal function in SLE patients as was done in several clinical trials.33-35 Moreover, CKD-EPI is more reliable than CrCl and the other equations, as our study describes. Rheumatologists can estimate renal function easily with the demographic data, standardized creatinine and with the help of internet or a smart phone (using CKD-EPI).
There are several limitations to this analysis. First, the study population was composed only of Mexican patients; however, the main objective of the current work was to find the best available creatinine-based equation, and, in this way, our results can be generalized. Finally, these equations were not tested for assessment of change in GFR over time, but this is a diagnostic study in which we wanted to clarify the best equation and not the change over time.
With our results, we suggest that the initial classification of patients with SLE should be with CKD-EPI, because of its reproducibility, ability to predict GFR in patients with low or high GFR, and ease of performance.
Acknowledgements
We are very grateful to Mr. John W Covey for his suggestions in manuscript preparation, and with Juan Ramón Torres Anguiano and Carlos Alberto Hernández Nieto for their participation in selection of patients.
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Figure 1. Difference between measured and estimated versus estimated GFR
Table 1. Equations for estimating GFR
Table 2. Clinical and demographic characteristics
Table 3. Phase 1. Precision, bias, and accuracy
Table 4. Phase 2. Precision, bias, and accuracy
Table 5. Sensitivity and specificity of each equation to classify patients as having or not GFR less than 60ml/min/1.73