Introducción: El acetato cálcico/carbonato magnésico (MgCO3) es un quelante de fósforo con ventajas en cuanto a coste, seguridad y tolerancia, con similar eficacia a la de otros fármacos. El objetivo del estudio es evaluar los efectos sobre el metabolismo fosfocálcico al sustituir hidróxido de aluminio [Al(OH3)] por MgCO3 en una cohorte de pacientes en hemodiálisis. Material y métodos: Se incluyen 21 pacientes con fósforo < 5 mg/dl, con Al(OH3) como único quelante. La conversión a MgCO3 se realizó sin variar el número de comprimidos. Se registraron características clínico-demográficas, tratamiento para hiperparatiroidismo secundario y parámetros analíticos antes de la conversión, y mensualmente durante cuatro meses. Resultados: La fosforemia disminuyó de 4,52 ± 0,99 a 4,02 ± 1,07 mg/dl (p = 0,027), con una reducción del producto calcio-fósforo de 40,20 ± 10,44 a 35,16 ± 11,06 mg2/dl2 (p = 0,037). No encontramos variaciones significativas en los niveles de calcio, hormona paratiroidea o 25-OH-vitamina D3. El número prescrito de comprimidos diarios se redujo de 3,33 ± 2,29 a 2,15 ± 2,21 (p = 0,020). Los tratamientos concomitantes no variaron. Observamos un aumento significativo inicial de la magnesemia de 2,21 ± 0,24 a 2,43 ± 0,39 mg/dl (p = 0,001), que posteriormente se mantuvo estable. Encontramos una disminución del aluminio sérico de 14,91 ± 8,55 a 8,47 ± 3,98 µg/l (p = 0,004), con niveles en rango recomendado en todos los pacientes. Conclusiones: El MgCO3 permite un buen control del fósforo sérico en pacientes en hemodiálisis previamente bien controlados con Al(OH)3, con menos comprimidos diarios. Se produce un ligero aumento en el magnesio sérico, sin significado clínico a corto plazo. Desconocemos los efectos de este aumento a más largo plazo.
Introduction: Calcium acetate/magnesium carbonate (MgCO3) is a phosphorus binder with advantages in terms of cost, safety and tolerance and it has a similar efficacy to other drugs. The objective of the study is to assess the effects of replacing aluminium hydroxide [Al(OH3)] with MgCO3 on phosphorus and calcium metabolism in a cohort of haemodialysis patients. Materials and methods: We included 21 patients with phosphorus <5mg/dl, with Al(OH3) as the only binder. The conversion to MgCO3 was carried out without changing the number of pills. We recorded clinical-demographic characteristics, treatment for secondary hyperparathyroidism and laboratory parameters before conversion and every month for four months. Results: Phosphataemia decreased from 4.52±0.99 to 4.02±1.07mg/dl (p=.027), and there was a decrease in the calcium-phosphorus product from 40.20±10.44 to 35.16±11.06mg2/dl2 (p=.037). We did not observe significant changes in levels of calcium, parathyroid hormone or 25-OH-vitamin D3. The daily number of pills prescribed was reduced from 3.33±2.29 to 2.15±2.21 (p=.020). Concomitant treatments were not altered. We observed an initial significant increase in magnesaemia from 2.21±0.24 to 2.43±0.39mg/dl (p=.001), which subsequently remained stable. We found a decrease in serum aluminium from 14.91±8.55 to 8.47±3.98µg/l (p=.004), with levels within the recommended range in all patients. Conclusions: MgCO3 allowed good control of serum phosphorus in haemodialysis patients who were previously well controlled with Al(OH)3, using fewer daily pills. There was a slight increase in serum magnesium, without short-term clinical significance. We do not know the effects of this increase in the longer term.
INTRODUCTION
Cardiovascular disease is the main cause of mortality in haemodialysis patients.1 Mineral metabolism disorders, and specifically hyperphosphataemia, are one of the factors directly involved through vascular calcification.2-5 As such, control of hyperphosphataemia and secondary hyperparathyroidism (SHPT) is one of the cornerstones of treatment in haemodialysis patients. Dietary restriction of phosphorus and appropriate dialysis are the first steps, but they are often insufficient and the prescription of phosphate binders is usually required.
Although the ideal binder has not yet been found, there are currently various drugs that are effective in terms of their main objective. However, there are differences in their pleiotropic and adverse effects and cost, and as such, the choice of binder must be rationalised and individualised for each patient.5-8
Aluminium hydroxide [Al(OH)3] has always been one of the most powerful binders. Its use has classically been restricted due to potential toxic effects, but there is no clear scientific evidence of these effects.9,10 We have used it regularly in our unit and have not observed higher serum aluminium levels than those recommended or related adverse events. Nevertheless, this binder has not been manufactured in recent years for commercial reasons.
One of the therapeutic options emerging in the market is the combination of calcium acetate with magnesium carbonate (MgCO3). Its advantages include a lower calcium intake than other calcium binder monotherapies and a lower cost than new non-calcium binders, with the same efficacy, as well as the potential benefits of additional magnesium intake.11
The objective of this study was to assess tolerance and efficacy in hyperphosphataemia control when we replaced Al(OH)3 (Pepsamar®) with MgCO3 (Osvaren®) in a cohort of haemodialysis patients.
MATERIAL AND METHODS
Patients
Between October and December 2012, we selected patients from our haemodialysis unit who had an adequate control of phosphataemia (serum phosphorus <5mg/dl), on treatment with Al(OH)3 binder monotherapy who required continuation of this treatment. We followed the indications on dietary restriction of phosphorus rich foods regularly and without variation, in accordance with the unit’s standard protocol. Twenty-one patients (66.7% male, aged 56.7±16.4 years) met the inclusion criteria. They all underwent dialysis in three four-hour weekly sessions; 6 received high-flux haemodialysis and 15 received online haemodiafiltration. Both the regimen and dialysate remained unchanged throughout the study, with there being a constant magnesium concentration of 0.5mmol/l. The dialysate calcium concentration did not change and was 1.25mmol/l in eight patients and 1.5mmol/l in the remaining 13. Patient baseline characteristics are displayed in Table 1. The conversion to MgCO3 was carried out without changing the number of pills or the time at which they were taken, which were indicated during or immediately after meals. The maintenance dose was adjusted to clinical criteria in accordance with monthly serum phosphorus levels.
Variables
We recorded the patients’ demographic and clinical characteristics. Before the conversion and over the following four months, we collected the data related to SHPT treatment each month, as well as laboratory parameters related to phosphorus and calcium metabolism: total and free calcium, phosphorus, 25-OH-vitamin D3, parathyroid hormone (PTH) and serum magnesium. Serum aluminium was measured at baseline and after four months. Patients were regularly questioned about their adherence to the treatment with phosphate binders.
Statistical analysis
All statistical analyses were performed using the SPSS version 17.0 software (SPSS Inc, Chicago, IL). The variables were recorded as percentages or as a mean ± standard deviation. Qualitative variables were compared using McNemar's test and quantitative data were analysed using the Wilcoxon signed-rank test for related non-parametric variables. We considered relationships with a P value <.05 to be significant.
RESULTS
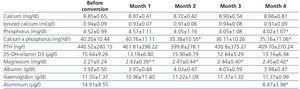
We observed a statistically significant decrease in serum phosphorus levels four months after the conversion to MgCO3 (4.52±0.99 versus 4.02±1.07mg/dl, p=.027), and in calcium-phosphorus product levels (40.20±10.44 versus 35.16±11.06mg2/dl2, p=.037). Despite the additional intake of oral calcium, the change in serum calcium was not significant (8.85±0.65 versus 8.66±0.81mg/dl). No significant changes were observed in PTH or vitamin D levels. Changes in laboratory results throughout follow-up are summarised in Table 2.
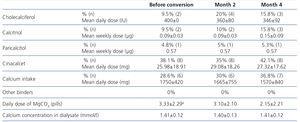
The improvement in phosphataemia control was achieved by decreasing the number of MgCO3 pills (from 3.33±2.29 to 2.15±2.21 pills per day, P=.020). There were no significant changes in the rest of the drugs used to treat SHPT (Table 3).
We discontinued treatment with MgCO3 in six patients (30%), in five due to hypophosphataemia and in one due to digestive intolerance. We observed a significant increase in magnesaemia from the first month (2.21±0.24 versus 2.43±0.39mg/dl, P=.001), which remained stable for four months. We recorded just one case of hypermagnesaemia (>3mg/dl), which was suitably resolved by reducing the dose.
Likewise, we found a decrease in serum aluminium levels from 14.91±8.55 to 8.47±3.98µg/l (p=.004), with levels being within the recommended range (<40µg/l) in all patients.
DISCUSSION
The conversion from Al(OH)3 to MgCO3 allowed phosphorus to be controlled adequately in this haemodialysis patient cohort. Although we were not able to demonstrate clearly that a decrease in serum phosphorus results in reduced mortality, the obvious pathophysiological role that it plays has led to clear indications in national and international guidelines.12
There are currently a wide variety of binders available, all of which are effective in reducing hyperphosphataemia, but with differences in other aspects that force doctors to make a decision about which binder to prescribe.13-15 Until recently, aluminium salts were considered the most powerful binder, with a very low cost/benefit ratio.11 However, the potential toxicity of aluminium had restricted its use in the literature to settings with fewer economic resources.16-18 There has even been a certain concern about aluminium intake from other drugs, both in terms of hidden intake and alongside other indications, especially antacids.19 Improvement in the treatment of dialysis water, with the resulting lower aluminium concentration, and its low cost in this period of adjustments has challenged these limitations. Various recent studies in which no long term toxicity was observed have reopened the debate on the potential use of aluminium salts.9,10,20,21
In our study, we found that suppressing aluminium salt-based binders effectively decreases serum levels of this element, although no patient had levels higher than 40µg/l at any given time. For now and until there is more evidence on the use of aluminium salts with the current water controls, the guidelines continue to advise against them. Furthermore, the manufacture and distribution of the aluminium binder available in our setting was recently interrupted, forcing us to seek other therapeutic options.
The purpose of our study was to assess the replacement of Al(OH)3 with MgCO3. The first studies in this line were carried out in the nineteen eighties, in different conditions to the current situation.22 Since then, many studies have used magnesium salts as phosphate binders.23 Of the many drugs available, MgCO3 has advantages in terms of safety and tolerance, with an efficacy that is comparable to that of other more modern binders.24 In our study, we found a significant reduction in the number of pills, which allowed an improvement in adherence and therefore in hyperphosphataemia control.25 It also has a lower cost than other non-calcium binders, which is important in the current situation.8
Another advantage of using MgCO3 is the benefits of magnesium intake, an element that is increasingly important.26 There is growing evidence of the relationship between lower levels of magnesium in the general population and the occurrence or poor control of diseases such as diabetes, high blood pressure or cardiovascular disease.27-31 In chronic kidney disease patients, reduced levels of magnesium have been associated with greater mortality, a worsening of mineral and bone disorder and an increase in vascular calcifications.32-35 An interventional study demonstrated delayed arterial calcification with a reduction in intima-media thickness in relation to magnesium supplements.36 At present, there are few studies on the effects of different dialysate concentrations.37,38 It seems that there could be certain advantages to using dialysate with a higher magnesium content, such as better haemodynamic tolerance.39 It is not clear what levels of serum magnesium are suitable, although there seems to be an increasingly greater consensus that somewhat higher levels could be beneficial for patients on dialysis. Intervention trials, which assess the medium and long term effects of increasing magnesium, whether through oral intake or higher dialysate concentrations, are necessary.
In our study, we observed a slight increase in magnesium levels, which, as in the CALMAG study, occurred at the start of treatment and subsequently remained stable.24 Only one case of asymptomatic hypermagnesaemia was recorded, which returned to levels below 3mg/dl after dose reduction. Likewise, we only found one case of digestive intolerance due to diarrhoea, which forced us to discontinue treatment.
Our study has various limitations: it is a non-controlled observational study, with a small patient sample and without strict control of adherence to a low phosphorus diet and binders. It is possible that due to the “study effect” patients will improve their level of adherence. Nevertheless, the changes observed in serum aluminium and magnesium suggest that there was at least partial adherence to treatment.
CONCLUSIONS
After the conversion from Al(OH)3 to MgCO3, there was adequate control of serum phosphorus and patients required a lower number of pills. We observed a slight increase in magnesaemia within the normal limits, whose long term clinical significance is as yet unknown. Prospective studies with a longer follow-up period are required for an accurate assessment of the long term effect of high levels of serum magnesium in patients on dialysis.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Patient baseline characteristics.
Table 2. Change in laboratory parameters after replacing aluminium hydroxide with calcium acetate/magnesium carbonate.
Table 3. Other treatments used for phosphorus-calcium metabolism disorders.