Introducción: La anemia es una complicación frecuente de la enfermedad renal crónica (ERC). El objetivo de este estudio fue conocer la prevalencia de anemia en pacientes con ERC estadios 3-5 no en diálisis atendidos en consultas externas (CCEE) de Nefrología en Cataluña y su manejo clínico. Metodología: Estudio epidemiológico, de cohorte transversal, multicéntrico, en condiciones de práctica clínica habitual. Recogida de datos mediante un e-CRD que incluía datos de filiación y aquellos relacionados con la anemia (hemoglobina, estatus férrico, tratamiento con agentes estimuladores de la eritropoyesis [AEE] y con otros coadyuvantes). Se definió anemia como unos niveles de hemoglobina < 13,5 g/dl en varones o < 12 g/dl en mujeres o pacientes que recibieran tratamiento con AEE. Resultados: Se incluyeron 504 pacientes (56,4 % varones, edad media de 67,8 ± 15,5 años): 61,5 % presentaban ERC estadio 3, 30,2 % estadio 4 y 8,3 % estadio 5. Las principales causas de ERC fueron la vascular y la nefropatía diabética. La prevalencia de anemia fue del 58,5 % (n = 295); sin embargo, solo un 14,9 % de los pacientes tenían niveles de hemoglobina < 11 g/dl. Los niveles medios de hemoglobina disminuían y el tratamiento con AEE era más frecuente a medida que progresaba la ERC, pero no se observaron diferencias significativas respecto a la prescripción de hierro, según estadios. Los AEE e intervalos más frecuentemente prescritos fueron darbepoetina alfa con una dosis mediana de 40 μg/bisemanal, seguida por C.E.R.A., con una dosis mediana de 75 μg/mensual y epoetina beta con una dosis mediana de 5000 UI/semanal. De los pacientes con anemia (n = 295), un 36,3 % (n = 107) presentaban ferropenia y de ellos solo un 53,3 % recibía tratamiento con suplementos de hierro. Conclusiones: Este estudio demuestra la alta prevalencia de anemia, la cual aumenta a medida que progresa la enfermedad, así como el buen control de la misma en la población de pacientes con ERC atendidos en CCEE de Nefrología en Cataluña. Este control se consigue con dosis moderadas de AEE y prescripción de suplementos de hierro en más del 50 % de los pacientes anémicos.
Introduction: Anaemia is a common complication of chronic kidney disease (CKD). The aim of this study was to determine the prevalence and clinical management of anaemia in patients with stages 3-5 CKD not on dialysis treated in outpatient Nephrology clinics (OC) in Catalonia. Methods: Epidemiological, cross-sectional cohort, multicentre study under routine clinical practice conditions. Data collection by electronic data collection log-book (e-DCL) including personal information and data related to anaemia (haemoglobin, iron status, treatment with erythropoiesis-stimulating agents [ESA] and other anaemia treatments). Anaemia was defined as haemoglobin levels <13.5g/dL in males or <12g/dL in females or patients who receive treatment with ESA. Results: We included 504 patients (56.4% male, mean age of 67.8±15.5 years): 61.5% had stage 3 CKD, 30.2% stage 4 and 8.3% stage 5. The main causes of CKD were vascular and diabetic nephropathy. The prevalence of anaemia was 58.5% (n=295), however, only 14.9% of patients had haemoglobin levels <11g/dL. Mean haemoglobin levels decreased and ESA treatment was more common as CKD progressed, but no significant differences were observed regarding the prescription of iron, according to CKD stages. ESA and intervals most frequently prescribed were darbepoetin alfa with a median dose of 40μg/biweekly, followed by C.E.R.A. with a median dose of 75μg/month and epoetin beta with a median dose of 5,000IU/week. Among the patients with anaemia (n=295), 36.3% (n=107) had iron deficiency and only 53.3% of these patients were treated with iron supplements. Conclusions: This study demonstrates the high prevalence of anaemia, which increases as the disease progresses and its good control in a CKD patient population treated in Nephrology outpatient clinics in Catalonia. This control is achieved with moderate doses of ESA and iron supplements prescribed in more than 50% of anaemic CKD patients.
INTRODUCTION
Chronic kidney disease (CKD) affects around 11% of the Spanish adult population, according to data from the EPIRCE study,1 and is associated with high morbidity and mortality, particularly as a consequence of cardiovascular disease.2,3 Furthermore, CKD is related to a series of complications, including anaemia,4 which has been linked to higher morbidity and mortality and CKD progression.
The presence of anaemia is already observed in early stages of CKD (stage 3), but its prevalence increases as renal failure progresses to more advanced stages.5 The main cause of anaemia in CKD patients is an erythropoietin deficiency, although a decrease in the half-life of red blood cells, iron and vitamin deficiency and other factors have also been involved.5
Since the introduction of recombinant human erythropoietin, erythropoiesis-stimulating agents (ESA) have become the cornerstone of CKD anaemia treatment and have reduced requirements for transfusion, improved the quality of life and reduced left ventricular hypertrophy and morbidity and mortality in these patients.6-10 However, target haemoglobin has been the subject of debate in recent years due to recent randomised studies and meta-analyses that have demonstrated that total anaemia correction with ESA is not associated with better survival or a significant improvement in quality of life, while it is associated with a potential increase in adverse cardiovascular effects.11-16
In light of these results, the 2010 European guidelines recommended target haemoglobin of between 11 and 12g/dl, without intentionally exceeding 13g/dl17 and the EMA (European Medicines Agency) advises not exceeding haemoglobin levels of 12g/dl in patients treated with ESA (Ref. EMEA/188068/2007).18
The objective of this study was to establish the prevalence of anaemia in patients with stages 3, 4 and 5 CKD not on dialysis treated in outpatient Nephrology clinics in Catalonia, and know its therapeutic management (haemoglobin levels achieved, prescription of ESA, iron therapy and other concomitant treatments) in clinical practice.
MATERIAL AND METHOD
Study design and data collection
This is an epidemiological, cross-sectional, multicentre study under routine clinical practice conditions.
The study data were collected during 2010. All Nephrology Services in Catalonia were invited to participate and we conducted a feasibility survey on all candidates. Nineteen centres were included in the study (90.47% of those invited), of which 17 recruited patients (80.95% of those invited). Two of the centres invited did not participate in the study. Participant centres contributed data from all patients visited consecutively in outpatient Nephrology clinics who met the inclusion and exclusion criteria and who gave their consent to participate in the study over a week, with the aim of including the highest possible number of patients. The data were collected using an electronic data collection log-book (e-DCL), which was developed specifically for the study. We compiled patients’ demographic data (age, gender, weight, height, CKD stage, CKD diagnosis time) and data corresponding to anaemia (haemoglobin levels, ferritin levels, transferrin saturation index [TSI], treatment with ESA, iron, folic acid or vitamin B12). We also estimated the glomerular filtration rate (eGFR) using the MDRD-4 formula.
The study was approved by the different ethics committees of participating hospitals and patients had to sign their informed consent to allow data to be taken from their clinical history.
Patient selection
The study’s target population was adult patients with CKD and an eGFR of less than 60ml/min/1.73m2 who were not on dialysis.
Inclusion criteria:
- Patients 18 years old or older.
- Patients with an established clinical diagnosis of stage 3, 4 or 5 CKD not on dialysis treated in outpatient Nephrology clinics in Catalonia.
- Patients who gave their informed consent in writing to participate in the study.
Exclusion criteria:
- Patients whose clinical history did not include a recent determination of haemoglobin (two months before inclusion).
- Any patient situation or condition that, in the opinion of the researcher, would make them unsuitable for participating in the study.
Statistical analysis
Anaemia was defined as haemoglobin levels <13.5g/dl in males and <12g/dl in females or treatment with ESA. We defined iron deficiency as ferritin levels <100ng/ml and/or TSI <20%.
Categorical variables were described using absolute and relative frequencies, including a 95% confidence interval. For the description of the continuous variables, we used means, standard deviation, medians, the minimum and maximum, including the total number of valid values.
For quantitative variables, we used parametric tests (Student’s t-test or Anova) or non-parametric tests (Mann-Whitney or Kruskal-Wallis), as appropriate. For qualitative variables, we performed the χ2 test.
The results were considered to be statistically significant when the P value was less than 0.05.
The statistical analyses were carried out using the SAS version 9.1.3 statistical software.
RESULTS
Study population
We recruited a total of 531 patients, of which we excluded 27 because they did not meet the selection criteria or did not have the data necessary for assessing the main variable, leaving 504 patients included in the study.
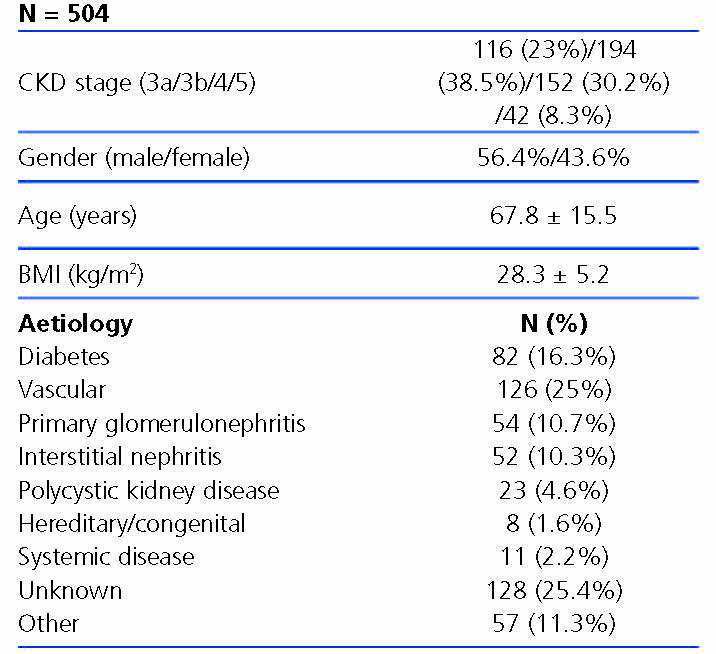
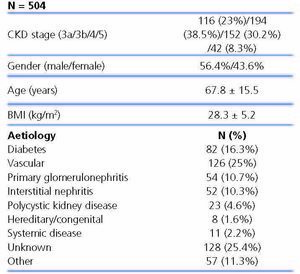
The characteristics of patients included are displayed in Table 1. 61.5% had stage 3 CKD, 30.2% had stage 4 CKD and the remaining 8.3% had stage 5 of the disease. The causes of CKD are displayed in Table 1. We observe that the main aetiologies are vascular and diabetic nephropathy, although the aetiologies of a high percentage of patients were unknown.
Laboratory data on anaemia and iron status
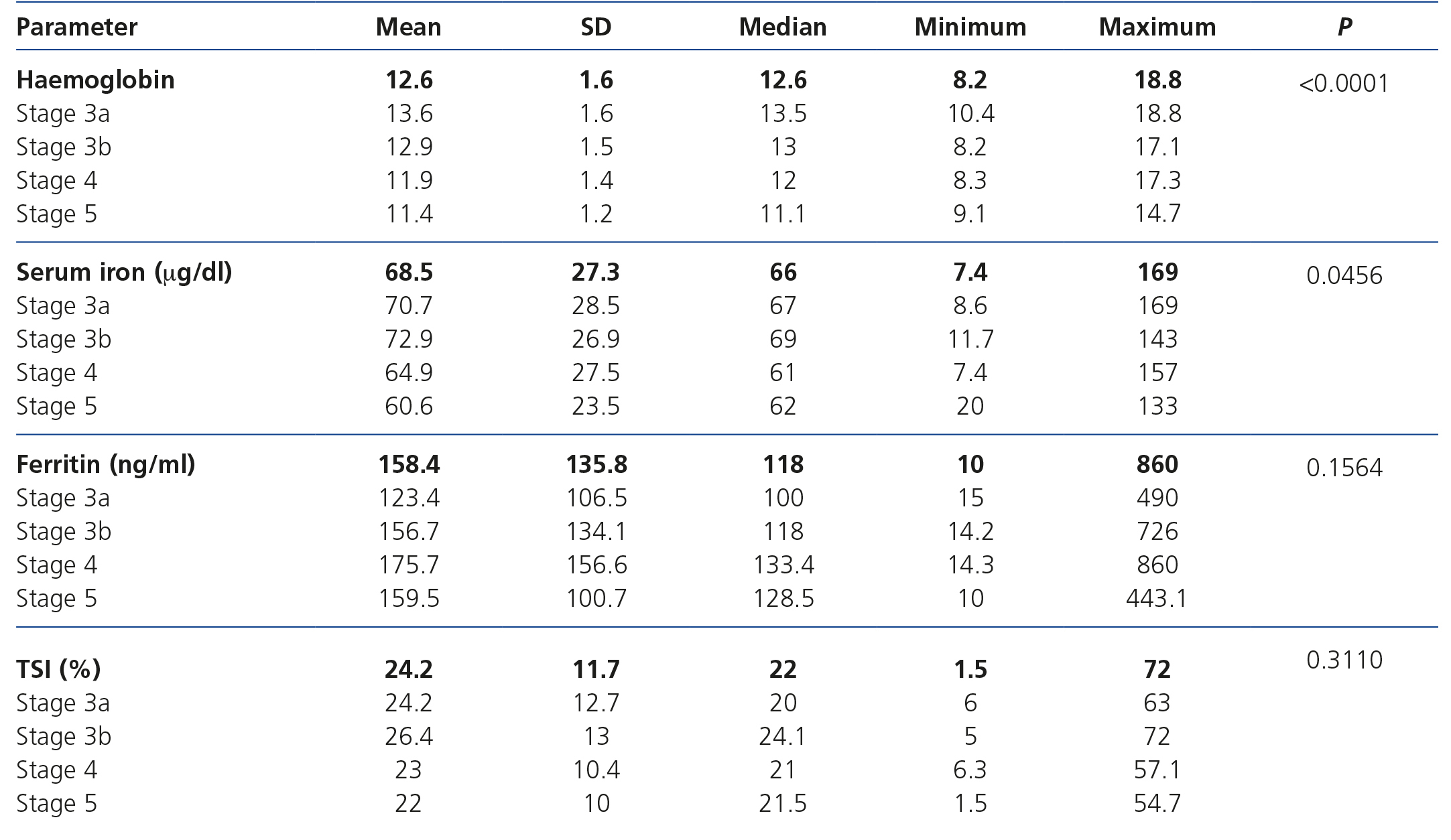
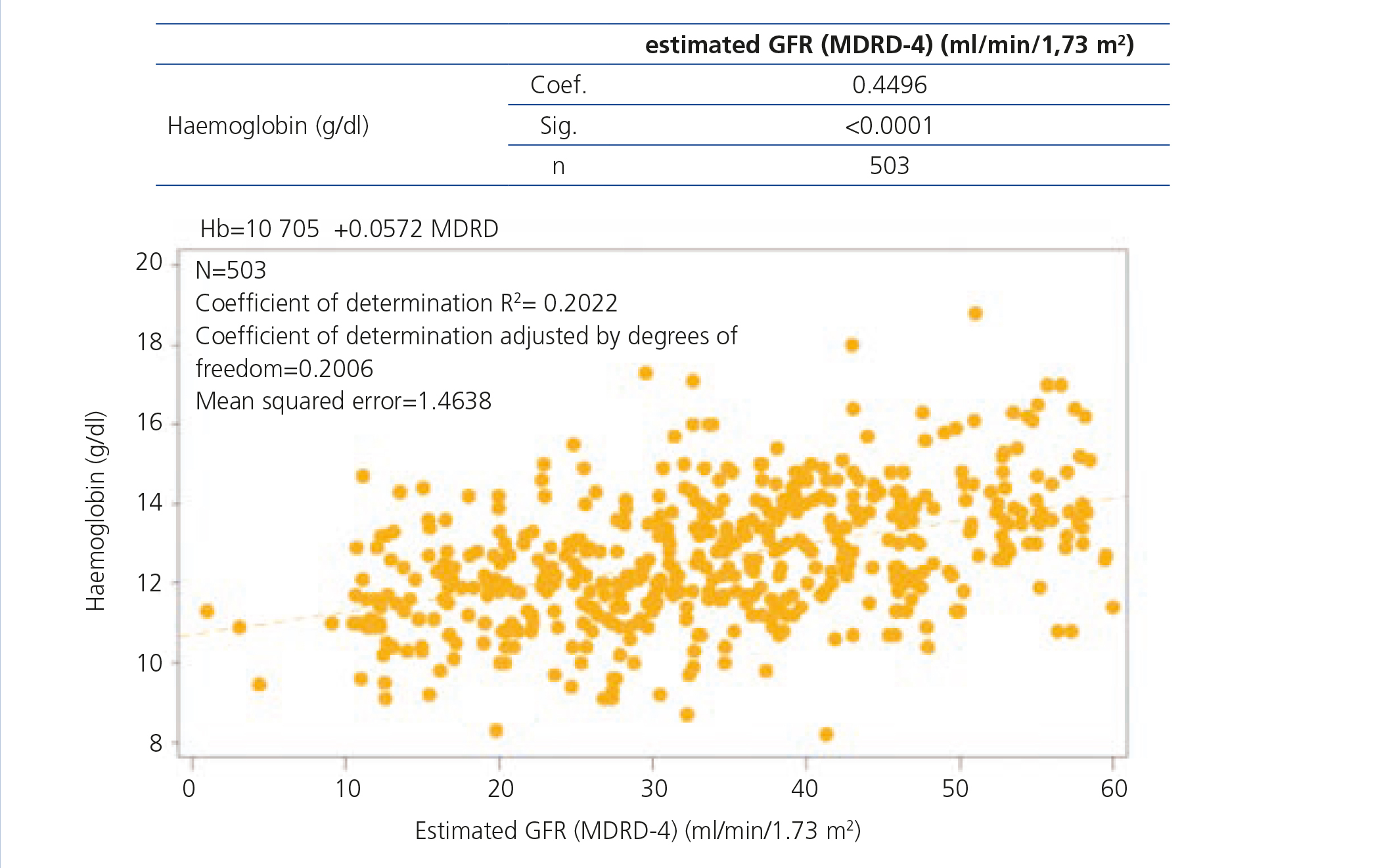
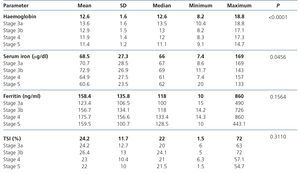
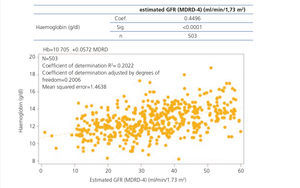
Data on haemoglobin and iron status are displayed in Table 2, which shows levels of haemoglobin, serum iron, ferritin and TSI according to CKD stage. Anaemia prevalence in the study population was 58.5%, although only 14.9% of patients had haemoglobin levels <11g/dl. The mean haemoglobin of the whole cohort was 12.6±1.6g/dl, although we observed a tendency for haemoglobin levels to decrease as CKD advanced. We observed an inverse correlation between the eGFR and haemoglobin levels (Figure 1). The mean ferritin of the whole study group was 158.4±135.8ng/ml and the mean TSI was 24.2±11.7%, without significant differences for ferritin or TSI being observed according to the stage of the disease. Among anaemic patients (n=295), 36.3% (n=107) had an iron deficiency (ferritin <100ng/ml and/or TSI <20%).
Anaemia prevalence according to demographic data
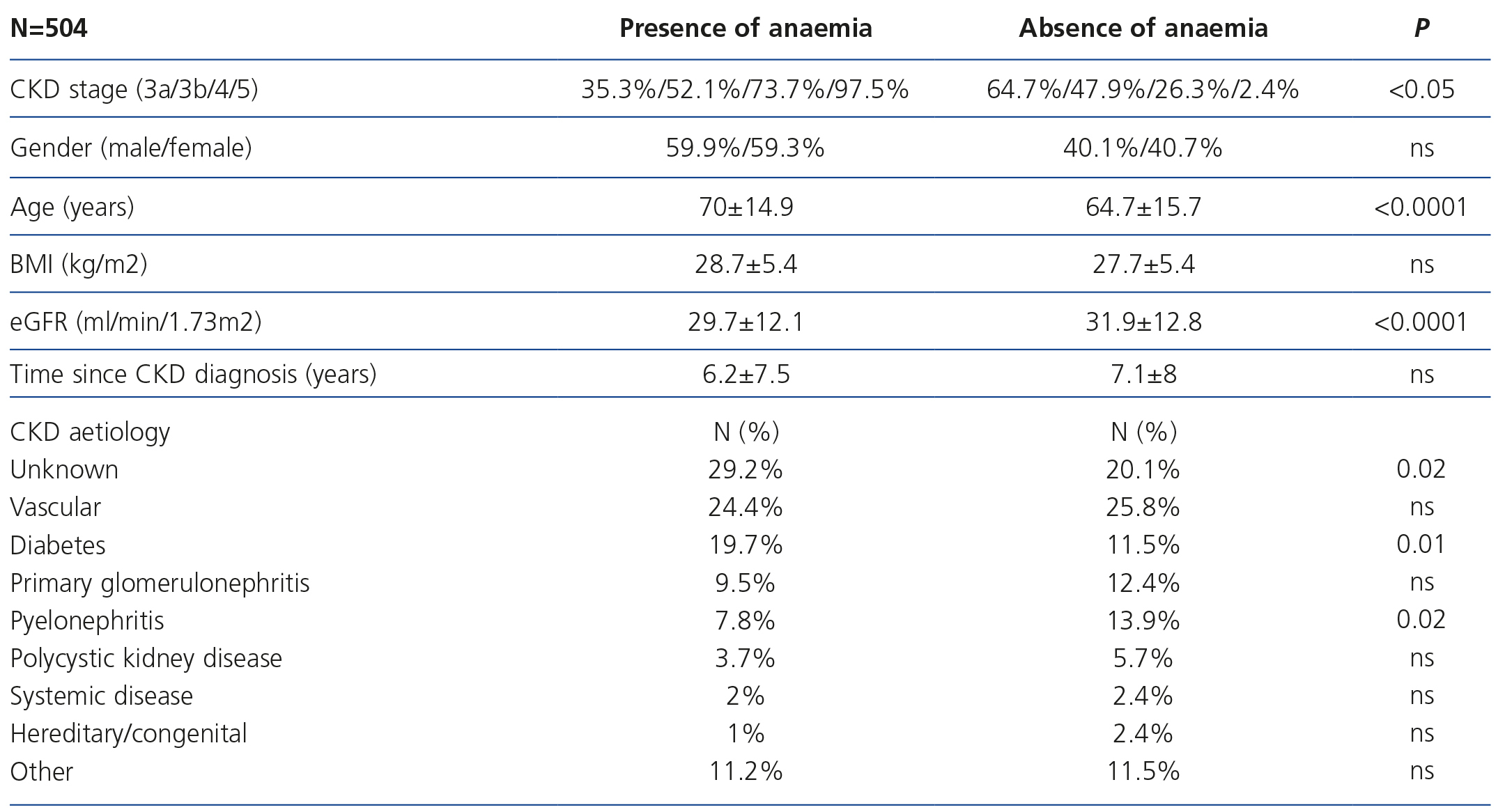
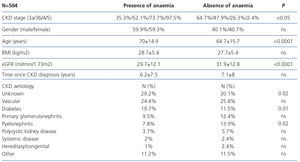
The patient’s profile according to the presence or absence of anaemia is displayed in Table 3, without significant differences being found according to sex, but with there being significant differences according to age (higher anaemia amongst older individuals), CKD aetiology (diabetes and unknown cause are the most prevalent aetiologies in anaemia), CKD stage (as CKD progresses, a higher presence of anaemia is observed) and eGFR (lower eGFR amongst patients with anaemia).
Treatments prescribed for anaemia
40.5% of all patients were receiving some form of treatment for anaemia at the time of the visit. The data referring to treatment for anaemia are displayed in Tables 4, 5, 6, 7 and 8.
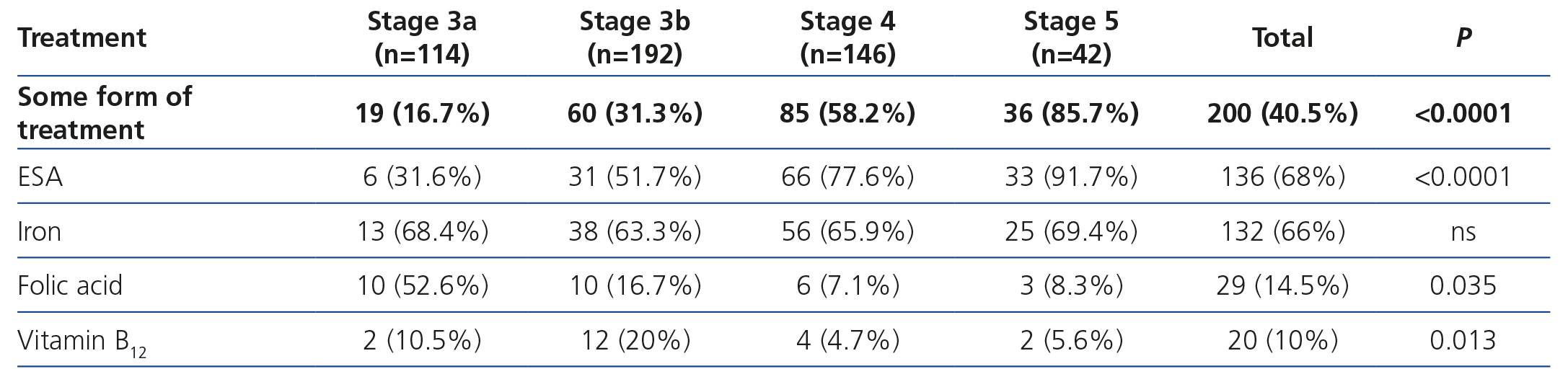
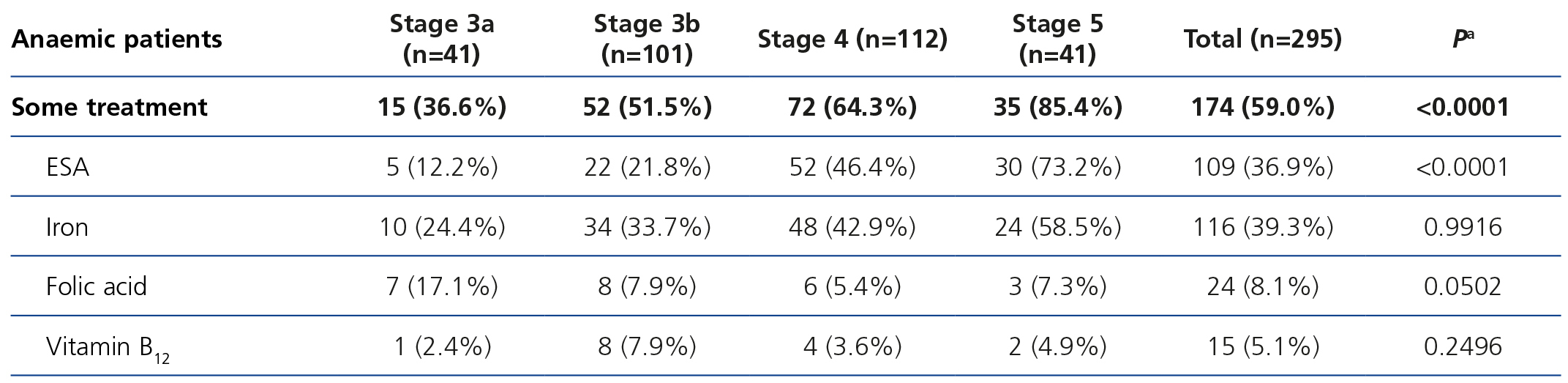
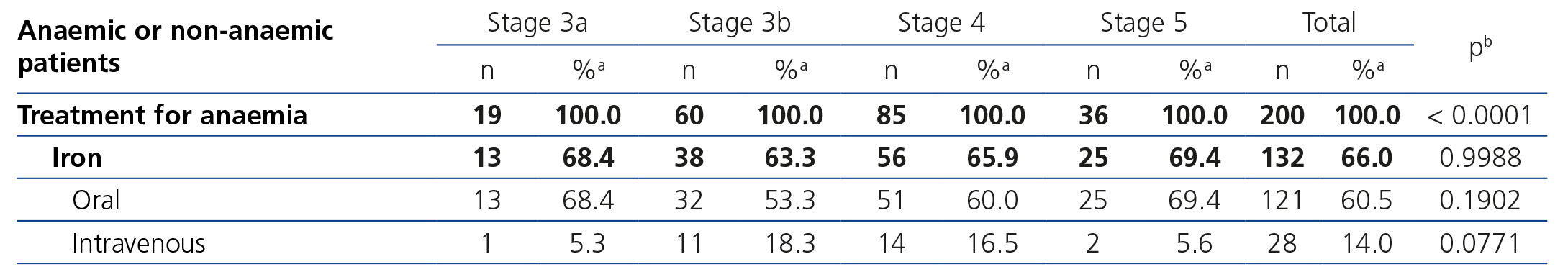
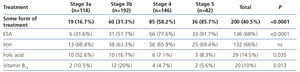
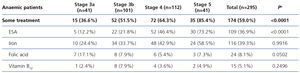
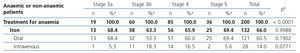
The treatments for anaemia are displayed in Table 4 according to the CKD stage. Of the patients treated, 68.0% (n=136) were receiving an ESA, 14.5% were receiving folic acid and 10.0% were receiving vitamin B12. 66.0% of all patients with chronic renal failure and anaemia were receiving iron supplements at the time of the visit (61% orally and 5% intravenously). We can observe how the percentage of patients treated increases as the CKD stage increases (Table 8). ESA was the most frequent treatment as CKD progressed, while folate and vitamin B12 were prescribed more often in earlier stages, but no significant differences were observed with regard to the prescription of iron supplements according to the stages. There were no statistically significant differences with regard to the prescription of oral and intravenous iron according to the CKD stage. In the anaemic patient subgroup we observed similar results to those of the overall population, although they were not significant for vitamin B12 and folic acid (Table 5). The ESA and intervals most frequently prescribed in our population were darbepoetin alfa with a median dose of 40μg/biweekly, followed by C.E.R.A (Continuous Erythropoietin Receptor Activator) with a median dose of 75μg/month, followed by epoetin beta with a median dose of 5000IU/week. The prescription of epoetin alfa to the study population was rare.
Table 6 displays treatment with iron supplements in the anaemic, iron-deficient patient subgroup; 53.3% received treatment with iron, 12.1% did not receive iron supplements and in the rest of the patients, it was not specified in the e-DCL. The prescription of iron to anaemic and iron-deficient patients was more common in patients with stage 5 CKD than in earlier stages.
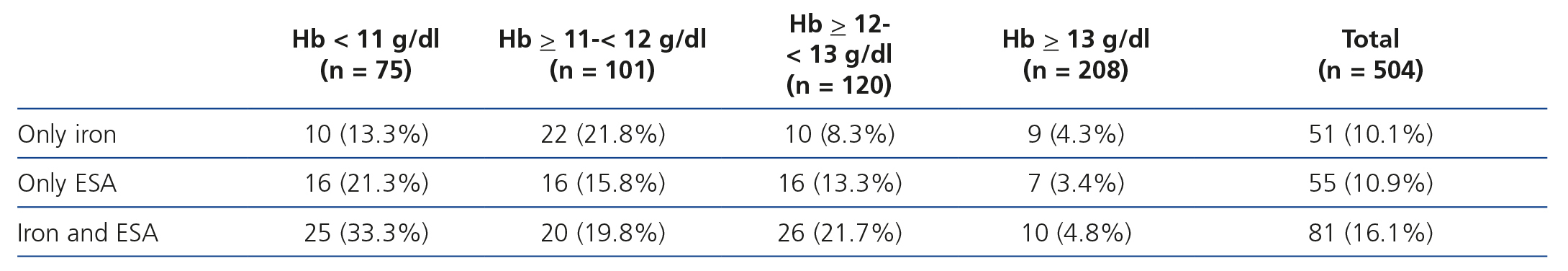
Table 7 displays treatment for anaemia according to haemoglobin levels, and we observed that 67.9% of patients with haemoglobin levels <11g/dl received ESA and/or iron therapy and 33.3% received both. Likewise, in patients with haemoglobin >12g/dl, 18 % received ESA, as well as 8.2% of those with haemoglobin >13g/dl. Of those treated with ESA, 43.4% had haemoglobin levels >12g/dl and 12.5% had haemoglobin levels >13g/dl.
DISCUSSION
This study shows a high prevalence of anaemia in patients with stages 3-5 CKD not on dialysis and a suitable control of the latter in patients treated in 17 Nephrology Service outpatient clinics in Catalonia.
The prevalence of anaemia in this patient population with stages 3-5 CKD not on dialysis was 58.5%, however only 14.9% had haemoglobin of less than 11g/dl, that is, that 85.1% of patients would exceed the low haemoglobin margin established by the European guidelines.17 In the MERENA observational multicentre study, conducted on 1129 patients with stages 3 and 4 CKD tested in outpatient Nephrology clinics in Spain, anaemia prevalence was somewhat lower than that of this study (51.3% compared to 58.5%), although it must be borne in mind that this study did not include patients with stage 5 CKD and it is possible that recently published referral criteria may have resulted in patients who are currently treated in outpatient Nephrology clinics having higher comorbidities, including anaemia, than in previous studies, such as MERENA.19 The prevalence of anaemia in 1058 patients with CKD not on dialysis in Italy was 16%, 32% and 51% in stages 3, 4 and 5 respectively, although the definition of anaemia in this study would require lower levels of haemoglobin (haemoglobin <12g/dl in males and <11g/dl in females).20 In a recent Spanish prospective study of 439 non-anaemic patients with CKD, followed up over three years, 35% developed anaemia, and they had a quicker progression of CKD and a higher risk of hospitalisation, cardiovascular events and mortality.21
Demographic factors that influence the presence of anaemia in the study population include CKD stage (greater prevalence of anaemia as the glomerular filtration rate decreases), age (greater prevalence of anaemia in older patients, probably related to a lower eGFR) and the aetiology of CKD (greater prevalence of anaemia in diabetic nephropathy and lower prevalence in interstitial nephritis). These results coincide with previous studies that demonstrate that anaemia is more common and severe at any eGFR level in diabetic patients, compared with non-diabetic patients.22,23
Mean haemoglobin of CKD patients was relatively high (12.6g/dl), bearing in mind that 40.5% of patients were receiving some form of treatment for anaemia. Of the 187 patients treated for anaemia, 72.7% (136 patients) were treated with ESA at the time of the study.
In earlier stages of CKD, only 16.7% of patients with stage 3a CKD and 31.3% with stage 3b CKD received some form of treatment for anaemia, with mean haemoglobin levels of 13.6g/dl and 12.9g/dl being observed, respectively. As CKD progressed, the percentage of patients who received some form of treatment for anaemia increased to 85.7% in stage 5 with mean haemoglobin of 11.4g/dl being observed.
In the most advanced stages of CKD (4 and 5), anaemia was controlled by more frequent use of ESA, notably ESA with a longer half-life, which allow them to be administered biweekly or monthly. This facilitates compliance, it is more comfortable for the patient and it improves haemoglobin stability. Furthermore, monthly doses of agents with a longer half-life (darbepoetin or C.E.R.A.) were lower than equivalent doses of erythropoietin, although given the cross-sectional nature of the study, these data should be interpreted with caution. It should also be highlighted from this study that anaemia control is achieved with relatively low doses of ESA (80μg/month darbepoetin, 75μg/month C.E.R.A.) with respect to other studies with darbepoetin or C.E.R.A.13,24-26 This is important because high doses of ESA have been associated with higher mortality27 and the guidelines advise considering the ESA doses necessary for achieving the haemoglobin levels.17
In our study, 43.4% of anaemic patients who received ESA had haemoglobin >12g/dl and 12.5% had haemoglobin >13g/dl, which suggests overtreatment with ESA in a significant percentage of them. Additionally in our study, we observed that 24 patients (32.0% of whom had haemoglobin <11g/dl) did not receive any treatment for anaemia.
Although the mean values of ferritin and TSI of the whole group were above the minimum values advised in the guidelines, 36.3% of anaemic patients had an iron deficiency. With regard to iron therapy, 53.3% of iron-deficient patients received treatment with iron supplements (most as oral iron supplements), 12% did not receive iron and there was no data available for 34.5%. Assuming that where no data was contributed the patients did not receive iron, this suggests an aspect of improvement in anaemia treatment in these patients, as indicated by recommendations by experts and the new KDIGO (Kidney Disease Improving Global Outcomes) guidelines, which promote iron therapy for optimising erythropoiesis and the response of the ESA treatment.28-29
The main limitation of this study for the interpretation of its results is its cross-sectional nature and that the conclusions are obtained from haemoglobin levels and iron parameters from just one laboratory test, and from the dosage of a treatment at one point in time. Another limitation concerns the difference between the valid definition for all the populations (haemoglobin <13.5g/dl in males and <12g/dl in females) and accepted target haemoglobin levels for good control of anaemia in CKD patients, defined as the result of the latest prospective multicentre studies carried out,11-13 which would be between 10 and 12g/dl, both in males and females. Furthermore, this study did not evaluate other parameters that influence haemoglobin levels and ESA dose, such as the presence of inflammation, nutritional parameters, treatment with angiotensin converting enzyme inhibitors or angiotensin II receptor antagonists, or intact parathyroid hormone levels. Furthermore, this study cannot be extrapolated to the general CKD patient population, since many are treated in Primary Care, but it can be extrapolated to the patient subgroup treated in outpatient Nephrology clinics, who presumably have more comorbidities, including anaemia.
The main conclusion of this study is that patients with stages 3, 4 and 5 CKD not on dialysis followed up in hospital clinics of Catalonia have a high prevalence but, in general, an adequate control of anaemia. This good control is achieved with a combination of moderate doses of ESA (particularly in advanced stages of CKD) and the prescription of iron in more than 50% of those who require it. Nevertheless, we have observed a significant percentage of patients treated with ESA with haemoglobin levels >12g/dl and a certain undertreatment of iron deficiency, which suggests that there are opportunities for improvement in the treatment of anaemia in our patients.
ACKNOWLEDGEMENTS
MICENAS I study researchers. The following researchers also participated in this study: Arias M. (Hospital Clínic, Barcelona), Perelló M. (Hospital Vall d´Hebron, Barcelona), Samon R. (Hospital Mollet, Barcelona), Agraz I. (Hospital Vall d´Hebron, Barcelona), Bayes B. (Hospital Germans Trias i Pujol, Barcelona), Cañas L. (Hospital Germans Trias i Pujol, Barcelona), Castellote E. (Hospital de Vic, Barcelona), Craver L. (Hospital Arnau de Vilanova, Barcelona), García R. (Hospital Palamós, Girona), Felip A. (Hospital Mataró, Barcelona), Lauzurica R. (Hospital Germans Trias i Pujol, Barcelona), Pou M. (Hospital Terrassa, Barcelona), Ballarín J. (Fundació Puigvert, Barcelona), Puig J.M. (Hospital del Mar, Barcelona), Puig C. (Hospital d'Igualada, Barcelona), Cao H. (Hospital del Mar, Barcelona), Collado S. (Hospital del Mar, Barcelona), Barbosa F. (Hospital del Mar, Barcelona), Martínez-Vea A. (Hospital Universitari Joan XXIII, Tarragona).
Conflicts of interest
This study was sponsored by the Societat Catalana de Nefrologia with financing from Roche Pharma in Spain.
Table 1. Characteristics of the patients included in the study.
Table 2. Laboratory data on anaemia (according to the different stages of chronic kidney disease).
Table 3. Patient profile according to the presence of anaemia.
Table 4. Treatment of anaemia according to the chronic kidney disease stage.
Table 5. Treatment of anaemia according to the chronic kidney disease stage in anaemic patients.
Table 7. Treatment for anaemia according to haemoglobin levels.
Table 8. Patients on treatment with oral iron and intravenous iron according to the chronic kidney disease stage.
Figure 1. Correlation between the haemoglobin level and the glomerular filtration rate.
12261_19157_56337_en_w47771409412ref.1226127659_12261_19115_54116_es_12261_tabla6_en.docx
Table 6. Treatment with iron supplements in anaemic and iron-deficient patients according to the chronic kidney disease stage.