To the Editor:
Arteriovenous fistulae (AVF) can be defined as “abnormal” connections between the venous and arterial systems that bypass the normal anatomic capillary bed. An AVF may be located in any area of the body and be congenital or acquired after some event (e.g. trauma). Lower limb fistulae are far more common acquired AVF, since the groin is a very common location for both arterial and venous vascular access.1-3
Predisposing factors include high blood pressure, overweight, anticoagulation or antifibrinolytic therapy, old age, the left side and female sex, as well as a history of punctures in the limbs.1,2 Labropoulos et al. reported the occurrence of AVF after episodes of deep vein thrombosis (DVT), particularly in large deep and proximal veins such as the femoral and popliteal veins. After the occlusion of an artery or vein, changes take place with the purpose of redirecting blood flow. In the case of arteries, the new circulation should have the purpose of providing oxygen and nutrition to organs and tissues; this arterial development is known as “collateralisation”. For veins, the adjustment is aimed at draining blood, and the term used to describe the new vein formation process is “neovascularisation”. While ischaemia is the stimulus for collateral formation, the mechanism responsible for venous neoformation is unclear. In any case, the fact that AVF particularly appear in proximal veins prompts us to ask whether low-resistance flow loss might be the stimulus for neovascularisation more than the thrombosis itself.4-6
Clinical assessment: in most cases of acquired AVF, there are no symptoms and if there are, they may appear within days or months and include thrill in the inguinal area, dyspnoea or new or worsening ischaemia in the limbs. Physical examination of the limb may reveal murmur, thrill, haematoma or pulsatile mass. Oedema, DVT, nerve compression or worsening of previous varicosities may also occur.
Diagnosis: Doppler is the preferred method of diagnosis, with angiography being used as a therapeutic tool for endovascular treatment.
Treatment: in small asymptomatic AVF, there is usually spontaneous thrombosis, and they do not require treatment, but treatment is indicated in cases in which symptoms appear. Currently, the techniques of choice are ultrasound-guided compression7,8 and percutaneous techniques, with surgery being reserved for selected cases, such as steal syndrome with claudication or significant distal ischaemia of the limb, oedema or venous insufficiency due to venous hypertension, heart failure due to AVF volume, AVF caused by stab wounds or fire and iatrogenic AVF that do not close spontaneously.
We report the case of a 58-year-old male with chronic kidney disease of unknown aetiology, on renal replacement therapy since December 2010. He was initially treated with peritoneal dialysis (PD) but was transferred to haemodialysis (HD) in December 2011. This patient had a spontaneous AVF in his left arm, which was eventually used as vascular access, with satisfactory results. The patient came to our clinic in 2007-2008, presenting with advanced renal failure (creatinine 3.5, estimated glomerular filtration rate 20ml/min; immunological study, tumour markers and serologies without findings and chronic ultrasound data). His medical history showed that he had high blood pressure, was a smoker and had an iliac blade fracture in 1998, after a fall, which required surgery. The physical examination was normal, except for the presence of an exophytic lesion on his upper lip, and he was diagnosed with moderately differentiated squamous-cell carcinoma of the supraglottic larynx and was treated with surgery and coadjuvant radiotherapy. He currently has no diseases and has a tracheostomy that is pending surgical closure. In December 2010, he started PD using a peritoneal catheter. After one month, he developed peritonitis due to Acinetobacter, which resolved with antibiotic treatment. After three months, he had another episode of peritonitis, due to Pseudomona aeruginosa, with several recurrences, which made it necessary to remove the peritoneal catheter and transfer the patient to HD. In February 2012, the patient received a new peritoneal catheter and on starting the technique, a peritoneal-pleural leak occurred with secondary massive pleural effusion, and he was permanently transferred to HD.
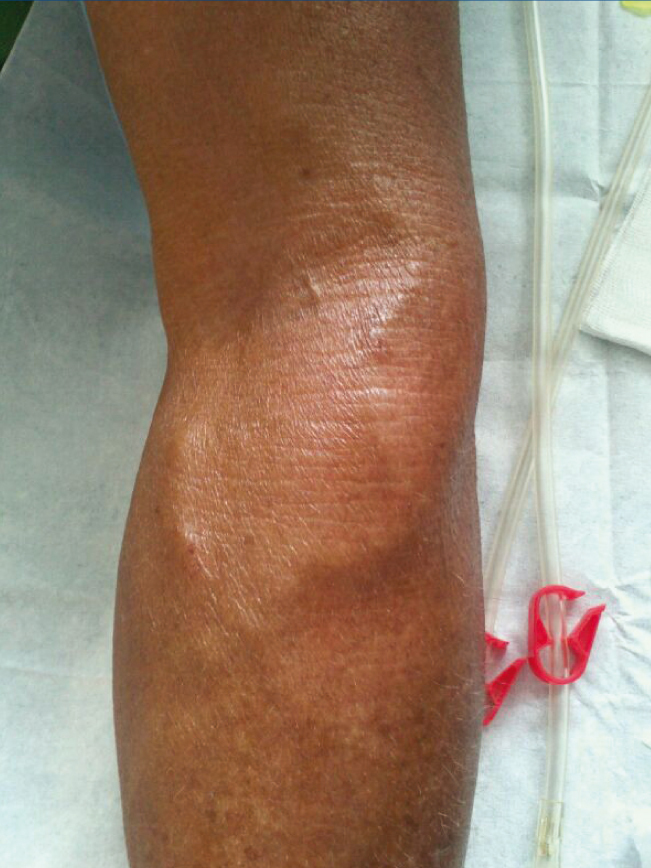
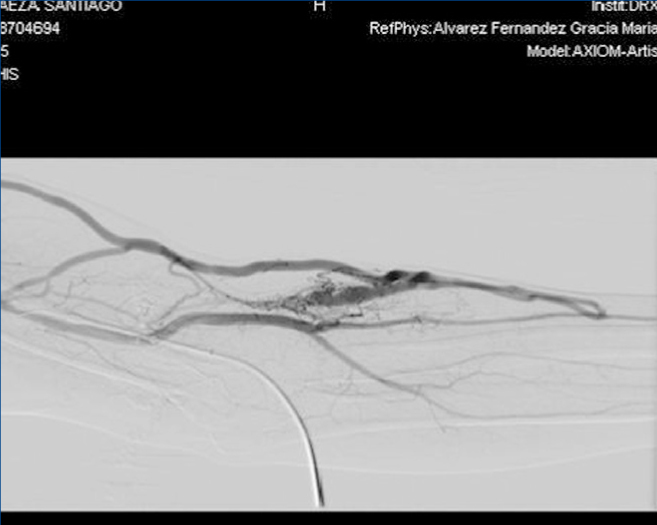
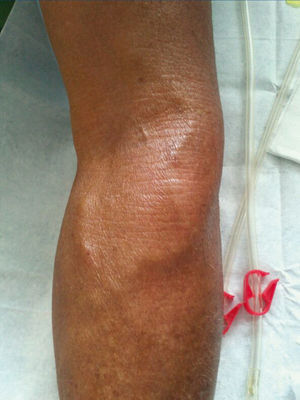
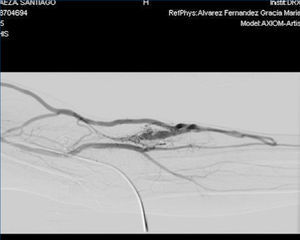
With regard to the vascular access, in November 2011, the patient received a left jugular dual lumen tunnelled catheter for HD. After six months, he presented with progressive oedema in his left arm, and a radiological study (venogram) was therefore requested, which displayed thrombosis in the patient’s left distal subclavian vein, brachiocephalic artery and left distal jugular vein. A thrombectomy was performed and we observed substantial improvement, although partial thrombosis remained in the brachiocephalic artery and left jugular vein. In the same operation, the jugular catheter was removed and replaced with a permanent left femoral catheter, with anticoagulation being introduced orally. A month after we detected thrombosis, in an operation to introduce an AVF, we detected a pulse and thrill in the patient’s left forearm, and after he was assessed by the vascular surgeon clinically and with a Doppler ultrasound of the arm, we found that the pulse and thrill were due to an AVF that was suitable for use in HD. A fistulogram was performed, which showed the presence of a humeral AVF, which was punctured without difficulties, with adequate suction and entry pressure, recirculation <10% and adequate dialysis doses; the situation remains the same at present.
The interest of this case lies in the fact that spontaneous AVF in the arms are extremely uncommon, and furthermore, in this case the AVF was very useful. Risk factors include high blood pressure, a history of numerous punctures for blood samples, brachiocephalic artery thrombosis and anticoagulation, initiated as a result of this.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Left femoral arteriovenous fistula.
Figure 2. Fistulogram.