Dear Editor,
Idiopathic inflammatory myopathies (IMM) constitute a rare group of systemic autoimmune conditions.1 Although their aetiology remains unknown, the chronic muscle inflammation probably results from a combination of genetic and environmental factors.2,3 Metabolic and infectious myopathies should be ruled out before accepting this diagnosis as definitive.4
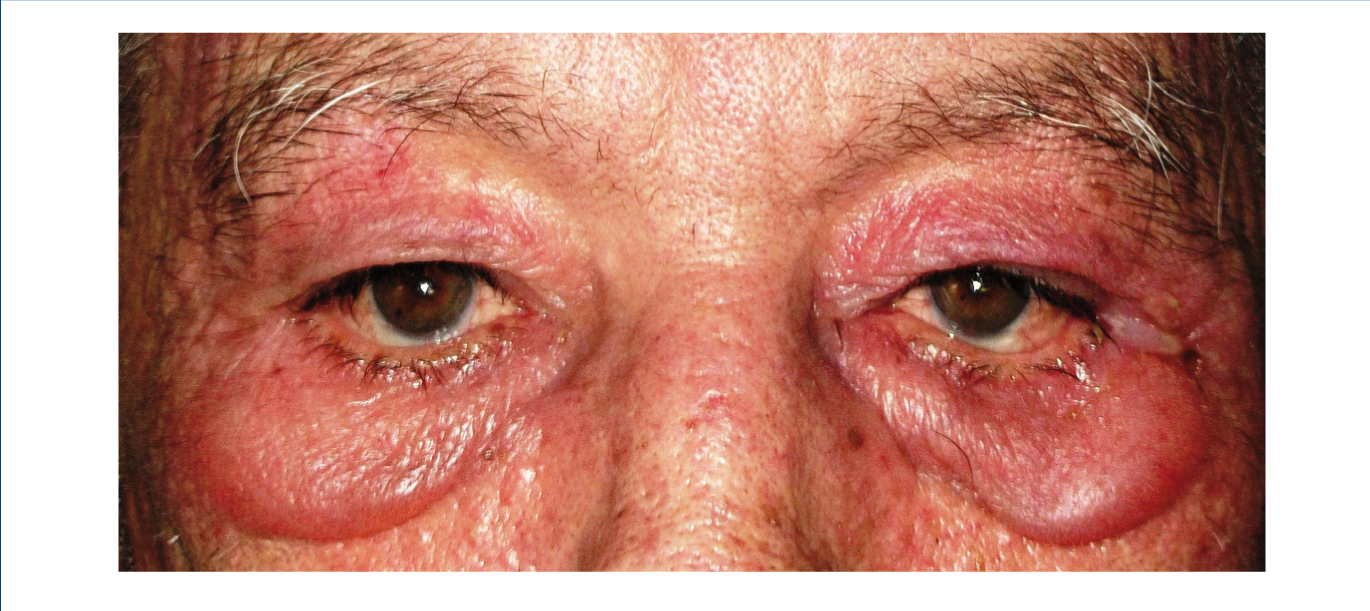
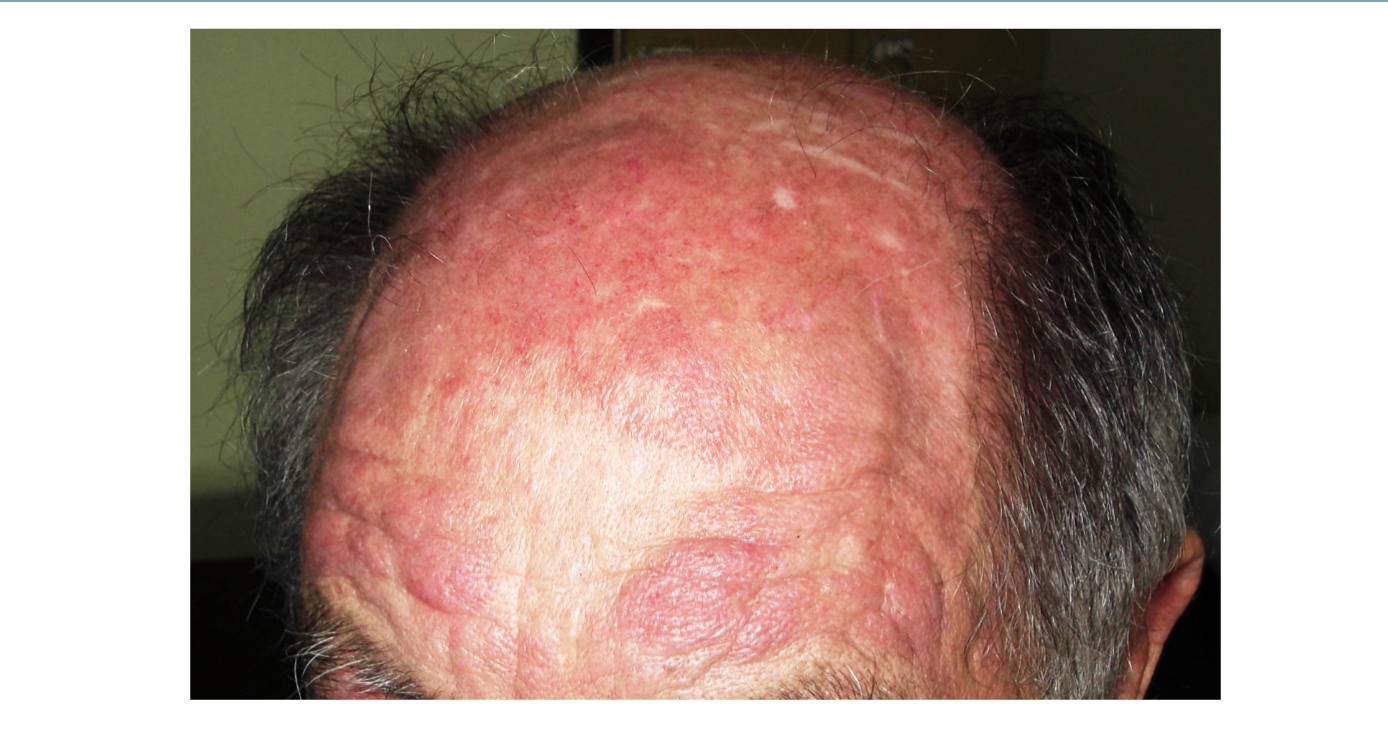
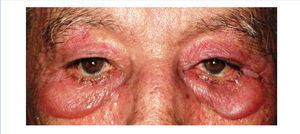
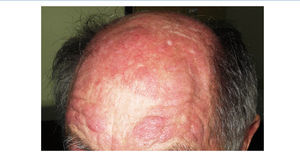
We report the case of a 76-year-old man with chronic kidney disease secondary to autosomal dominant polycystic kidney disease, submitted to a cadaveric kidney transplant in 1992 and with normal allograft function, referred to the Nephrology Department with complaints beginning one month before consisting of asthenia, anorexia, weight loss and non-specific tenderness in both thighs, associated with symmetrical proximal muscle weakness in the upper and lower limbs. His medical history was significant for monoclonal gammopathy of undetermined significance diagnosed five months earlier, with no signs of progression to overt myeloma, and subclinical hypothyroidism, medicated with levothyroxine and appropriately controlled. No history of recent trauma or unusual physical activity was identified. There was no personal or family history of known autoimmune or muscle diseases. Further chronic medication included azathioprine 50mg id, CsA 75mg bid, prednisolone 5mg id, furosemide 40mg id, omeprazole 20mg id and dipyridamole 75mg bid. He denied intake of other pharmaceutical drugs (particularly statins), illicit drugs or herbal therapies. Physical observation revealed unremarkable cardiac, pulmonary and abdominal examinations. Slight generalized erythroderma, periorbital oedema with heliotrope rash (Figure 1), psoriasiform changes of the scalp (Figure 2) and Gottron papules were evident upon close inspection. Neurological exam revealed generalized muscular atrophy and proximal muscle strength grade 4/5. Laboratory results at admission revealed normal-range haemoglobin, white blood cell count, platelets, blood urea nitrogen, creatinine, serum electrolytes, thyroid and parathyroid hormone studies, and albumin. Creatine phosphokinase (CPK) was increased at 1,319U/L (normal <171U/L), as was alanine transaminase (ALT) 63U/L (normal <45U/L), aspartate aminotransferase (AST) 123U/L (normal <35U/L), lactate dehydrogenase (LDH) 317U/L (normal 125-220U/L), aldolase 14.9U/L (normal <7.6U/L) and C-reactive protein 0.56mg/dL (normal <0.5mg/dL). Urinalysis was normal. Viral serologies were negative for hepatitis B surface antigen, hepatitis C antibody, Human Immunodeficiency Virus 1 and 2 antibodies, Epstein-Barr virus and cytomegalovirus antigenaemia. Azathioprine was discontinued due to presumed drug-induced hepatotoxicity, while CsA and prednisolone were continued. Persistently elevated CPK (>1,000U/L) and other muscle enzymes, progressively worsening clinical symptoms after five days of optimal hydration status and diuresis, and onset of dysphagia and dysphonia directed an investigation for inflammatory myopathies. Complement levels were normal and autoimmunity testing revealed positive antinuclear antibodies (+), normal anti-double-stranded DNA antibodies, positive anti-cytoskeleton fibres antibodies (+++) and positive anti-vimentin antibodies (+++), with negative myositis-associated antibodies (Jo-1, Mi-2, Ku, PM-Scl 100, SRP, PL-7, PL-12, EJ, OJ and SSA52). Electromyography displayed evidence of muscle fibre lesion in the proximal muscles of the lower limbs, characterised by normal insertional and spontaneous activity, but presence of polyphasic motor unit action potentials of short duration and low amplitude. Skin biopsy was compatible with dermatomyositis. Muscle biopsy revealed no mononuclear inflammatory infiltrates, but intense and diffuse immunoexpression of Major Histocompatibility Complex (MHC) class I in the sarcolemma of studied tissue fibres. Studies undertaken for secondary causes were negative and there was no evidence of interstitial lung disease on respiratory function tests. Oral prednisolone was increased to 1mg/kg/day and he was started on daily physiotherapy. An optimal clinical and laboratorial response (at discharge: CPK 75U/L, ALT 36U/L, AST 27U/L, LDH 226U/L, aldolase 7.8U/L) permitted discharge after 51 days of hospitalization. He is currently on prednisolone 5mg (weaned over nine months) and developed steroid-induced diabetes mellitus with need for insulin therapy. His muscle strength is much improved, with no evident skin alterations, dysphagia or dysphonia.
A common set of criteria for the diagnosis of dermatomyositis and polymyositis was proposed by Bohan and Peter in 1975, where four of five conditions are necessary for a definitive diagnosis: (i) characteristic skin findings, (ii) proximal muscle weakness, (iii) elevated muscle enzyme levels, (iv) abnormal electromyography, and (v) abnormal findings on muscle biopsy.5 In our patient, all criteria were fulfilled to diagnose dermatomyositis. Regarding the classification scheme proposed by the 119th European Neuromuscular Centre international workshop,6 our patient falls in the probable dermatomyositis category by not fulfilling the perifascicular atrophy criterion on muscle biopsy. Classic histological findings, especially identification of mononuclear cellular infiltrates, remain the basis of the diagnosis, however these can be absent even in the presence of disease. MHC class I expression on the sarcolemma has been proven to be upregulated in IMM and is a valid test for the diagnosis of these myopathies since they are not affected by potential sampling errors.7 Common myositis-specific autoantibodies present with a frequency ranging from 1-30% and were negative in this case, but are not required for definitive diagnosis of inflammatory myopathies. There is an increased risk for malignancies,5 and age-appropriate cancer screening tests should be performed. Major therapeutic aims include elimination of muscle inflammation, restoration of muscle strength and prevention of chronic muscle disease in order to reduce morbidity and improve quality of life.5 Immunosuppressive therapy entails corticosteroids at a dose of 1mg/kg/day.5,8 Concurrent administration of methotrexate, azathioprine or mycophenolate mofetil may be started,5 but may be reserved for patients who fail treatment with glucocorticoids alone. In retrospect, we believe that the patient´s worsening symptoms during the earlier stages of hospitalization were actually due to the discontinuation of azathioprine, which was probably responsible for ameliorating the underlying condition. Due to the improved clinical and biochemical status with the increased dosage of prednisolone and physiotherapy, in conjunction with presumed azathioprine-induced liver toxicity, we selected not to resume azathioprine, and the patient currently remains asymptomatic.
This case reflects an interesting case of dermatomyositis, since the concept of an inflammatory or autoimmune myopathy seems difficult to fathom in a patient on regular immunosuppressive therapy for the past 20 years, particularly with a regimen including corticosteroids, CsA and azathioprine.
Long-term follow-up data on patients with IMM are scarce. Studies on the pathophysiology of IMM in patients on chronic immunosuppressive regimens are warranted.
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Figure 1. Heliotrope rash.
Figure 2. Psoriasiform scalp.