We present the case of a 76-year-old patient with primary Sjogren's syndrome (pSS) and mild acute renal failure with active sediment. Prior medical history included hypertension well controlled with 2 drugs and pSS with glandular (biopsy) and extraglandular involvement (pulmonary fibrosis, skin purpura and Raynaud's disease that required treatment with steroids), being treated with azathioprine, prednisone 5mg/day, biphosphonate, calcium/vitamin D, pentoxifylline, acetylcysteine, perindopril 4mg and amlodipine 5mg.
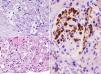
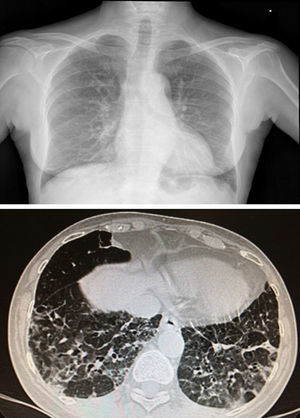
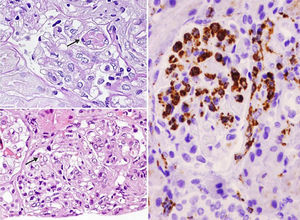
She was admitted in July 2013 because of recurrence of purpura in the lower limbs, weakness, fever of 37.8°C, dry cough, nausea and bilious vomiting, together with weight loss of up to 10kg in 2 months. Findings on physical examination included skin purpura and bilateral basal hypoventilation, attributed to her pulmonary fibrosis. She was stable as confirmed by CT scan (Fig. 1), with no palpable lymphadenopathies. Findings on laboratory testing included acute normochromic normocytic anaemia that was neither haemolytic nor iron-deficient, with normal protein electrophoresis, creatinine 1.07mg/dl (reatinine clearance of 68ml/min), proteinuria 200mg/dl (0.38g/24h) and sediment 20 RBCs/field (56% dysmorphic). Positive routine autoimmunity (ANA+, Ro+, La+, C4 hypocomplementaemia 1mg/dl and very high IgM and rheumatoid factor [RF]). In addition to current C3 hypocomplementaemia 66mg/dl, drop in IgG 600mg/dl (previously normal) and positive for cryoglobulins. Negative hepatitis C and B and HIV serology. Negative ANCAs. A skin biopsy was performed and reported small-vessel leukocytoclastic vasculitis. A renal biopsy of 28 glomeruli reported the following: glomerulomegaly; in 3 glomeruli, widespread sclerosis and, in the rest of glomeruli, lesions of ischaemia–basement membrane folding, mesangial and endocapillary hypercellularity, and some small PAS+hyaline thrombi with inflammatory cells CD68+ in their interior; mononuclear interstitial infiltrate; mild fibrosis and atrophy; focal tubular inflammation and ischaemia; arterioles with focal deposition of PAS+hyaline substance and thickening of the intima on immunofluorescence (9 glomeruli/1 sclerotic); IgM+ in interstitial inflammatory cell foci and around tubules; C3± heterogeneous mesangial C3 and in vascular walls; and negative Epstein–Barr virus in situ hybridisation (Fig. 2).
Chest X-ray and CT scan. Distortion of the pulmonary interstitium on the subpleural level of both bases, with thickening of the intralobular interstitium and interlobular septa, consistent with pulmonary fibrosis predominately on the posterior–basal subpleural level. Also bilateral basal cylindrical bronchiectasis.
Renal biopsy. Glomeruli with mesangial and endocapillary proliferation in which some hyaline pseudothrombi of different sizes are observed (arrows). (A) Periodic acid–Schiff (PAS) 60×. (B) Haematoxylin and eosin 40×. (C) Presence of numerous intraglomerular macrophages. Immunohistochemistry for CD68 40×.
Given such findings, she was diagnosed with mixed cryoglobulinaemia associated with pSS, and it was decided to start treatment with rituximab 375mg/m2/week (4 doses) and to maintain azathioprine and prednisone at routine doses. The result was satisfactory: CD20 lymphocytes dropped to 0.3%, the anaemia and renal profile (sediment and clearance) returned to normal and she was negative for cryoglobulins. At her last follow-up (25 months post-biopsy), the stability of renal function and percentage of CD20 B lymphocytes persisted, but she was again positive for cryoglobulins at her last 2 follow-ups. Now, we are expectant, with close monitoring.
Notable in this case was the low clinical expressivity of the patient's cryoglobulinaemic glomerulonephritis, which we attributed to early clinical diagnosis. The indication for a renal biopsy was relative, given the mild nature of the clinical and laboratory findings, but baseline disease and the large variety of potential associated nephropathies1 led us to perform the renal biopsy. Had it been delayed, she probably would have had greater parenchymal damage.
The most commonly reported finding in kidney biopsy of pSS is tubulointerstitial disease; glomerular disease is rare and coexistence of the two as in the patient described is uncertain. The presence of the glomerular and cryoglobulinaemic component seems to worsen the prognosis of survival.1,2
Induction with Rituximab in cryoglobulinaemic glomerulonephritis produces positive results in terms of the balance efficacy/safety.3 The baseline level of CD27+ memory lymphocytes seems to predict a better response to this drug.4 With respect to maintenance treatment, nothing has been established yet. A relapse rate of up to 40% has been reported, but this was in series with a majority of patients with HCV+ and without consideration of predictive factors.5 Reactivating the complement, cryoglobulins or CD20 cells does not in itself is not an indication of a new cycle of immunosuppressant therapy, but it suggest a closer follow-up as a precaution in anticipation of a clinical recurrence. This is the attitude adopted in this case.
Please cite this article as: Martín-Gómez MA, Caba Molina M, Cruz Caparros G, Muñoz Vico J, Gómez Morales M. Síndrome de Sjogren y nefropatía mixta. La importancia de la precocidad en la biopsia renal. Nefrologia. 2016;36:451–453.