Sacubitril/valsartan reduces cardiovascular morbidity and mortality in patients with systolic dysfunction (SD). The aim of the present study was to assess the evolution of chronic kidney disease (CKD) patients after initiating sacubitril/valsartan.
MethodsWe included 66 consecutive CKD patients with SD followed up in outpatient care. Patients had to meet the inclusion criteria of having a New York Heart Association class II to IV, receiving maximum tolerated doses of optimal medical therapy and CKD stages 1–4. At baseline, comorbidities and epidemiological data were collected and low doses of sacubitril/valsartan were initiated. At month 1 and 3, doses of sacubitril/valsartan were increased up to the maximum doses if tolerated. In each visit, renal function and cardiac biomarkers were recorded. All the data were analyzed at the end of follow up (6 months).
ResultsOf the 66 patients, 42 (63 %) were men, with a mean age of 73±15 years. Mean creatinine at baseline was 1.42±0.5mg/dl (glomerular filtration rate (GFR) estimated by CKD-EPI was 50±19ml/min/1.73m2) and mean left ventricular ejection fraction (LVEF) was 31±9 %. At the end of follow up, LVEF improved from 31±9%–39±15 % (p<0.001). After one month of treatment, renal function improved up to 53±21ml/min/1.73m2, p=0.005. For the remaining follow-up time, GFR remained stable (mean at end of follow-up 51±18ml/min/1.73m2). Seven patients (10.6 %) withdrew from treatment.
ConclusionIn our experience, sacubitril/Valsartan is safe in CKD, offering stability in CKD progression after 6 months.
Sacubitrilo/valsartan ha demostrado ser eficaz en la reducción de la morbi-mortalidad cardiovascular en los pacientes con disfunción sistólica (DS). El objetivo del presente estudio fue analizar la evolución de pacientes con enfermedad renal crónica (ERC) tras el inicio de sacubitril/valsartán.
Material y métodosSe incluyó a 66 pacientes consecutivos que acudieron a las consultas externas de Nefrología, con ERC y DS. Los criterios de inclusión fueron: presentar una clase funcional II a IV de la New York Heart Association (NYHA) con el tratamiento médico optimizado y ERC estadios 1 a 4. Se recogieron datos basales epidemiológicos y de comorbilidad en el momento de inicio del fármaco. En los meses 1 y 3 se tituló la dosis de sacubitril/valsartán (en función de la tolerancia). En cada visita se recogieron datos analíticos de función renal y biomarcadores cardiacos, entre otros. Se analizaron los datos a los 6 meses (fin del seguimiento).
ResultadosDe los 66 pacientes, 42 eran varones (63%) con una edad media 73±15 años. La creatinina media fue de 1,42±0,5mg/dL (filtrado glomerular CKD-EPI 50±19ml/min/1,73m2) con una fracción de eyección del ventrículo izquierdo (FEVI) media de 31±9. Al final del seguimiento, la FEVI mejoró significativamente (basal 31±9 vs final 39±15, p<0,001). En cuanto a la función renal, el filtrado glomerular por CKD-EPI presentó mejoría al mes (50±19 vs 53±21ml/min/1,73m2, p=0,005) que se mantuvo estable (filtrado glomerular al final del seguimiento 51±18ml/min/1,73m2). Abandonaron el tratamiento 7 pacientes (10.6%).
ConclusiónEn nuestra experiencia, sacubitril/valsartán es seguro en los pacientes con insuficiencia renal crónica y estabiliza la función renal a los 6 meses.
Patients with chronic kidney disease (CKD) have a high risk of cardiovascular events to the point that CKD is being included as a cardiovascular risk factor per se.1 The situation is more severe in advanced CKD; studies in large populations have shown that the worse the impairment in glomerular filtration, the more likely to suffer cardiovascular events.2
In addition, heart failure (HF) is a clinical situation that is accompanied by high morbidity and mortality. Different factors, including acute myocardial infarction or pathological dilation of the left ventricle impair cardiac function and the ejection fraction is not maintained (systolic dysfunction or HF with reduced ejection fraction) or defects of myocardial relaxation during ventricular filling (diastolic dysfunction or HF with normal ejection fraction).3
Both situations, CKD and HF, may be the cause and the consequence of each other, thus their deleterious effects are enhanced and constitute the so-called cardiorenal syndrome.4
To date, the treatment of HF had been based on the use of angiotensin-converting enzyme inhibitors in combination with beta blockers or even aldosterone antagonists. Recently, neprilysin inhibition has acquired a relevant role in the treatment of HF, by demonstrating (in combination with valsartan) its ability to decrease hospitalizations and even mortality in patients with reduced left ventricular ejection fraction (LVEF) in patients that had optimized medical treatment.5,6 Presently, the indication of sacubitrile / valsartan includes patients who with a functional class ii-iv of the New York Heart Association (NYHA) classification with optimized medical treatment and LVEF below 40 %, although the ongoing study PARAGON is designed to evaluate the efficacy of the drug in HF patients with normal ejection fraction.7
With regard to renal function, clinical trials have included patients with renal insufficiency (glomerular filtration rate [GFR] < 30mL / min / 1.73m2) although only the UK HARP-III had as its main objective the evaluate the change in GFR. In this study, there was a similar reduction in GFR and proteinuria after 12 months of follow-up with both treatment (irbesartan vs. sacubitrile/valsartan).8
Beyond clinical trials, experience with this drug in every day practice has hardly been reported. For this reason, the objective of this study is to analyze the impact of sacubitrile/valsartan in patients with CKD.
MethodsThis is a retrospective cohort study including all consecutive patients who were prescribed sacubitrile/valsartan in the outpatient nephrology clinic during 2017. As Inclusion criteria: older than 18 years, with CKD stages 1–4 and with clinical criteria to receive treatment following to current recommendations.9,10 According to the guidelines at the initiation of the study, sacubitrile/valsartan was recommended to replace for angiotensin-converting enzyme inhibitors, in patients with HF with reduced ejection fraction (less than 40 %) who persisted symptomatic (NYHA class II-IV) despite receiving the optimal treatment (including beta blockers and aldosterone inhibitors at maximum tolerated doses).11 Exclusion criteria, were those derived from the contraindication of the drug prescription, hypotension defined as systolic blood pressure less than 100mmHg or serum potassium levels greater than 5.4mmol/L. The study included patients seen in 2017 and a follow-up extended for 6 months. The study was terminated on June 30, 2018.
Baseline information included: demographic data (age and sex), medical history such as hypertension (according to the definition of 8°report of the Joint National Committee12 or use of at least one antihypertensive), dyslipidemia according to the ATP III guidelines (LDL cholesterol higher than 160mg/dL in patients with 0–1 risk factor; greater than 130mg/dL in patients with more than one risk factor; greater than 100mg/dL in patients with coronary heart disease or equivalent or in treatment with hypolipidemic agents13), diabetes mellitus,14 history of cerebrovascular events, ischemic heart disease (defined as a history of acute myocardial infarction or angina) and echocardiographic parameters (LVEF [by Teichholz], pulmonary hypertension, diastolic dysfunction, defined by the quotient of the wave E / A or E / e ', if available15). The concomitant medication and its modification during patient follow-up wasalso collected.
The dose of sacubitrile/valsartan was gradually titrated. It was started with 24/26mg every 12h, then 49/51mg every 12h at the 1st month and finally, 97/103mg every 12h at 3 months, providing that it was tolerated. Patients were evaluated at the beginning of the study and in the subsequent visits at 1, 3 and 6 months of treatment. In these visits, blood and urine was collected for analytical determination to assess renal function (creatinine, estimated GFR [FGe] by CKD-EPI, albumin/creatinine ratio [ACR] in urine). Serum Sodium, potassium, uric acid and N-terminal peptide natriuretic factor (Nt-proBNP)16,17 were also determined. In addition, at 6 months, data from echocardiogram performed by protocol was collected. During the follow-up, adverse events and treatment abandonments were recorded.
The study complies with current data protection regulations, as well as ethical principles and it was approved by the Ethical Committee of the University Hospital of La Princesa. Patients expressed their consent to participate in the study.
Statistic analysisThe variables are expressed as mean±standard deviation if normally distributed (as the Kolmogorov-Smirnov) or median (interquartile range) otherwise. As it was a paired study, the Student t-test was used for paired samples (when quantitative variables were compared with qualitative ones), the McNemar test (to compare 2 qualitative variable variables) or ANOVA for repeated measures (to compare 2 quantitative variables). The comparisons were made taking as reference the baseline value (prior to the start of the medication).
All statistical analyzes were performed with the SPSS program (SPSS Inc., Chicago, IL, USA) version 21.0. A p<0.05 was considered statistically significant.
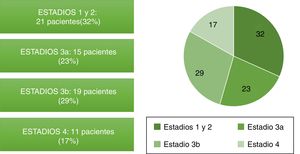
ResultsBaseline characteristicsThere were 66 patients included, of which 42 (63 %) were male. The average age was 73±15 years. A 80 % of patients (53) had a history of ischemic heart disease, and the mean LVEF was 31±9 %. Parameters of renal function, at the time of inclusion, were : mean serum creatinine: 1.42±0.50mg/dL; eGFR: 50±19mL/min/1.73m2. The ACR was only available in 23 patients and the median was 6 (2–48) mg/g. The etiology of CKD was distributed in: 37 (56 %) patients with nephroangiosclerosis, 17 (26 %) patients with diabetic nephropathy and 12 (18 %) of unknown etiology. The rest of the baseline characteristics are shown in Table 1. Fig. 1 shows the distribution of patients' CKD stages.
Baseline characteristics of the patients.
| Variable | Distribution n (%) |
|---|---|
| Sex (male) | 42 (63) |
| Age (years) | 73±15 |
| Hypertension | 64 (97) |
| Dyslipidemia | 46 (70) |
| Diabetes | 34 (51) |
| History of stroke | 7 (10) |
| Ischemic heart disease | 5 3 (80) |
| Pulmonary hypertension | 30 (45) |
| Diastolic dysfunction | 28 (41) |
| LVEF (%) | 31±9 |
| Basal Cr (mg/dL) | 1.42±0.5 |
| CKD-EPI (mL/min/m2) | 50±19 |
| ACR (mg/g)a | 6 (2.8–48) |
ACR: albumin/creatinine ratio in urine; Cr: creatinine; LVEF: left ventricular ejection fraction.
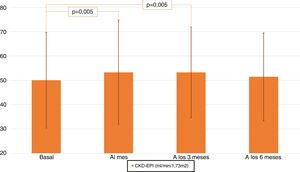
Renal function improved significantly after the first month (eGFR for baseline CKD-EPI 50±19 vs. 53±21mL/min/1.73m2 ; p=0.005) and continued to be improved the 3rd month (eGFR by CKD-EPI 53.3±18.7mL/min/1.73m2; p=0.005 from baseline) to it decreased at the sixth month (51,4±18mL/min/1.73m2 ; p=NS with respect to baseline) (see Table 2 and Fig. 2). It was analyzed the evolution of eGFR in the different CKD stages and it was found that CKD satge 3b patients improved eGFR (mL/min / 1.73m2) significantly throughout the follow-up (baseline 37±3, first month 39±6, third month 40±10 and sixth month 40±8 ; p=0.048 between baseline and third and sixth month).
Analytical evolution during follow-up after starting sacubitril/valsartan.
| Basal | Month 1 | Month 3 | Month 6 | |
|---|---|---|---|---|
| Creatinine (mg/dL) | 1.42±0.50 | 1.38±0.54a | 1.34±0.46a | 1.37±0. 53 |
| eGFR per CKD-EPI (mL/min/1.73m2) | 50.0±19.7 | 53.3±21.5a | 53.3±18.7a | 51.4±18 |
| Sodium (mmol/L) | 141±3 | 142±3 | 142±3 | 141±3 |
| Potassium (mmol/L) | 4.5±0.5 | 4.5±0.5 | 4.5±0.5 | 4.6±0.5 |
| Uric acid (mg/dL) | 6.9±1.7 | 6.6±1.7 | 6.9±1.6 | 6.8±1.4 |
| Albumin/creatinine in urine (mg/g) | 6 (2–48) | 11 (3–46) | 13 (4–37) | 8 (3–48) |
| Nt-proBNP (pg/mL) | 2485 (1,278-3934) | 1691 (816-3935)a | 1,520 (697-3208) | 1589 (581-3278) |
Values expressed as mean±standard deviation or median (interquartile range).
eGFR: estimated glomerular filtration; Nt-proBNP: N-terminal cerebral natriuretic peptide.
Regarding albuminuria, only in 23 patients had quantifiable values and no variation was demonstrated before and after the treatment (Table 2).
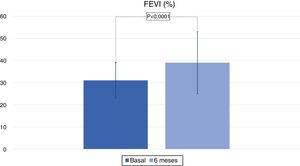
Patients had a significant improvement LVEF, which was of 39±15 % (p<0.0001) at the end of the study (Fig. 3). There were no significant changes in serum concentrations of sodium, potassium or uric acid (Table 2). The Nt-proBNP decreased significantly (p<0.001) the first month and there were maintained lower the rest of the follow-up (Table 2).
Dose at 3 months follow-upThe dose of sacubitrile / valsartan was evaluated at 3 months follow-up. As shown in Table 3, the dose distribution was similar for stages 1 to 3a and the maximum dose was reached by 43 % of the patients. In more advanced stages, only one patient reached the full dose. At 6 months, 18 patients reached the maximum dose.
Dropouts and adverse effectsDuring the follow-up, treatment was discontinued in 7 patients (10 %). Four patients (6 %) required hospital admission. The reasons for discontinuation of treatment were the onset of renal replacement therapy (a patient with stage 3 CKD who discontinued the drug at 2 months,), arterial hypotension (a patient with stage 2 CKD who suspended the drug at the 1st month), acute renal failure (2 patients: one of them with a stage 3a CKD suspended the drug at 1 month and another with stage 3b CKD at 3 months), diarrhea (a patient with stage 4 CKD who suspended the therapy a month), acute lung edema cardiogenic (one patient, stage 3a and who discontinued the drug at 3 months) and loss of follow-up (a patient who did not attend the clinic visit one month after starting treatment and had CKD stage 2). No other adverse effects were detected.
MedicationA 74.4 % (49) of the patients had prescribed beta blockers at the initiation of the study, 77.3 % (51) were on furosemide and 43.9 % (29) on spironolactone. After the first month, the dose of furosemide was reduced in 11 patients (16.7 %) at the discretion of the prescribing physician.
DiscussionIn the present study, based on real everyday clinical practice, it is demonstrated that the use of sacubitrile/valsartan is safe in patients with CKD and generates a renal benefit in terms of progression of renal failure. As a novelty and in contrast to published studies, our study demonstrates the beneficial effect of the drug in a population of older patients with a higher percentage of diabetes.
The treatment of HF has undergone a significant change with the accessibility to sacubitrile/valsartan, initially known as LCZ696. The benefit of this molecule lies in the ability of sacubitrile to inhibit the action of neprilysin, an endopeptidase that degrades vasoactive substances such as natriuretic peptides, bradykinin or adrenomodulin.18 Although theoretically sacubitrile may reduce blood pressure and improve HF by such mechanism (especially through the inhibition of natriuretic peptides), it was necessary to associate valsartan after demonstrating that an escape in the activation of Angiotensin II secondary to the inhibition of neprilysin limited its effect, especially with regard to the effect on blood pressure.19
Results from randomized clinical trials have been able to demonstrate the superiority of sacubitrile/valsartan as compared with enalapril in reduction mortality and hospitalization due to HF with reduced LVEF. In the PARADIGM-HF study, 8442 patients were randomized to receive this drug and it was compared with enalapril in subjects with HF stages IIIV of NYHA, with systolic dysfunction despite optimized medical treatment. Patients with CKD were included, although the limit was a eGFR of 30mL/min/1.73m2. In addition to demonstrating the superiority of sacubitrile/valsartan over enalapril in cardiovascular terms, the change in renal function and the appearance of hyperkalemia were analyzed as secondary objectives; deterioration of renal function was more frequent in the enalapril group (although the definition of this event was too weak: patients that reached creatinine above 2.5mg/dL).5 In our study, we have been able to demonstrate that renal function not only remains stable but even transient improvements may occur although not clinically significant. It is likely that this was due to the drug's ability to improve the HF functional class, which allows adjustement the diuretic treatment (in our case, these were 11 patients), in addition to the positive hemodynamic effect on the glomerulus itself. In unpublished data we have been able to verify that in these patients the extracellular volume (analyzed by spectroscopic bioimpedance) improves after the initiation of the drug, allowing a fine adjustment in the dose of loop diuretics. In a post hoc analysis of the PARAMOUNT-HF study (which included patients with HF with preserved ejection fraction) it was shown that after 36 weeks of treatment, sacubitrile/valsartan improved GFR, but with an increase in proteinuria when compared with valsartan monotherapy.20 Although in our study only 23 patients had quantifiable proteinuria, we found no differences in proteinuria during follow-up. In the recent study, UK HARP-III, which randomized 414 patients with GF 20−60mL/min/1.73m2 to receive sacubitril/valsartan versus Irbesartan. The main objective of the study was to evaluate the non-inferiority of the first versus the second treatment. The results demonstrated, no inferiority of sacubitril/valsartan versus Irbesartan in the stability of renal function and albuminuria.8 Regarding the potassium values, in our study they remained stable, without changes in the medication such as type of ion exchange resin or the potassium-sparing drugs.
Regarding the dosage, the patients who reached the highest dose were those with a better eGFR, as seen in Table 2. In the TITRATION study, 540 patients were randomized to receive 2 dose escalation regimes to assess tolerability (one conservative and one condensate).21 Although no differences were described in terms of renal function or hyperkalemia in both groups, better treatment adherence was observed in patients who received the most gradual dose escalation.
During the follow-up, an improvement in the LVEF was demonstrated in patients who underwent a control echocardiogram at 6 months, also of the values of Nt-proBNP were reduced with the treatment. Recently, the PIONEER-HF study has demonstrated the ability of sacubitrile/valsartan to decrease Nt-proBNP values in hospitalized patients with HF as compared to enalapril.22,23 It should be remembered that, unlike BNP, the inhibition of neprilysin does not alter the Nt-proBNP values and, therefore this reflects overload of the left ventricle.24
As far as adverse effects and dropouts (10 %), they were similar to those published in clinical trials. The most notable adverse effect in the clinical trials is hypotension and the increase in serum potassium levels.5 However, in our case only one patient abandoned the treatment due to hypotension and there were no adverse events due to increased potassium.
Our work is not exempt from limitations, such as the small sample size, and being perfomed in a single center or the retrospective design itself, a reason why not all data was collected at all times. However, we consider that the results reflect everyday clinical experience with the drug, therefore, they should be taken into account.
ConclusionsIn conclusion, the data from our study complement those observed by previous clinical trials but our population was older and with a higher prevalence ofdiabetes. Sacubitrile / valsartan is safe in patients with renal impairment and even offers some degree of renal function improvement. We did not find arterial hypotension or any relevant ionic alterations in patients treated with sacubitrile/valsartan.
Conflict of interestsBQ and VA have participated in lectures paid by Novartis.
Please cite this article as: Quiroga B, de Santos A, Sapiencia D, Saharaui Y, Álvarez-chiva V. Experiencia clínica con sacubitrilo/valsartán en pacientes con insuficiencia renal: la visión del nefrólogo. Nefrologia. 2019;39:646–652.