Overhydration (OH) complicates frequently the clinical course of Peritoneal Dialysis (PD) patients, and keeps a controversial association with the risk of peritoneal infection. The main objective of this study was to disclose an association between persistent OH and the risk of enteric peritonitis in a relatively large sample of patients undergoing PD.
MethodFollowing a prospective design, we monitorized systematically body composition of patients treated with PD in our unit (2011–2016), searching for a correlation with the ensuing risk of peritonitis, with an emphasis on the association between persistent OH (main study variable) and the risk of infection by enteric pathogens (main outcome). Essential demographic, clinical and laboratory variables with a potential influence on the risk of peritonitis were recorded. We used multivariate survival analysis to clarify the specific effect of different body composition parameters on the main outcome.
Main resultsWe included 139 patients for analysis (mean follow-up 24 months). Sixty-three patients suffered at least one peritonitis, and 17 had at least one diagnosis of enteric peritonitis. Univariate analysis disclosed a general trend to an increased risk of enteric peritonitis in overhydrated patients, as evidenced by associations of this outcome with mean extracellular water/intracellular water (ECW/ICW)(p = 0007), OH/ECW (p = 0033) and ECW/total body water (ECW/TBW)(p = 0004) ratios, but not with absolute OH values. Multivariate analysis confirmed similar associations or trends (RR 3,48, 95 % CI 1,03–14,59, p = 0,046, highest versus lowest tertile of ECW/ICW, RR 2,31, 95 % CI 0,98-6,56, p = 0,061, highest versus lowest tertile of OH/ECW, and RR 6,33, 95 % CI 1,37-19,37, p = 0,011, highest versus lowest tertile of ECW/TBW). On the contrary, no apparent association was detected between OH and the overall risk of peritoneal infection.
ConclusionPersistent overhydration portends a significant risk of peritoneal infection by enteric pathogens, among patients undergoing chronic PD.
La sobrehidratación (SH) es frecuente, y a menudo persistente, en pacientes tratados con Diálisis Peritoneal (DP), y mantiene una asociación controvertida con el riesgo de infección peritoneal. El objetivo principal de este estudio fue develar una posible asociación entre la presencia de SH y el riesgo subsiguiente de infección peritoneal por gérmenes entéricos, en una población relativamente amplia de pacientes tratados con DP.
MétodoSegún diseño prospectivo, monitorizamos de manera sistemática la composición corporal de pacientes tratados con DP en nuestra Unidad (2011–2016), buscando una posible correlación con el riesgo de peritonitis durante el seguimiento, con un interés particular en la asociación entre SH persistente (variable de estudio principal) y el riesgo de infección peritoneal por patógenos entéricos (variable resultado principal). Para el análisis tuvimos en cuenta variables demográficas, clínicas y de laboratorio con influencia potencial en el riesgo de infección peritoneal. Utilizamos técnicas de análisis multivariante para clarificar el efecto específico de diferentes parámetros de composición corporal sobre la variable resultado principal.
Resultados principalesIncluímos 139 pacientes, con seguimiento medio de 24 meses. Sesenta y tres pacientes sufrieron al menos una peritonitis, y 17 al menos una infección por gérmenes entéricos. El análisis univariante mostró una tendencia general a mayor riesgo de infección peritoneal entérica en pacientes sobrehidratados, que se hacía evidente cuando se usaba el cociente agua extracelular/agua intracelular (AEC/AIC)(p = 0,007), el cociente SH/AEC (SH/AEG)(p = 0,033), o el cociente AEC/agua corporal total (AEC/ACT)(p = 0,004), pero no cuando se usaba la sobrehidratación absoluta, como variable de estudio. El análisis multivariante confirmó estas asociaciones o tendencias (RR 3,48, IC 95% 1,03-14,59, p = 0,046, tercil mayor versus menor para AEC/AIC, RR 2,31, IC 95% 0,98-6,56, p = 0,061, tercil mayor versus menor para SH/AEC, y RR 6,33, IC 95% 1,37-19,37, p = 0,011, tercil mayor versus menor para AEC/ACT). Por el contrario, no observamos asociación consistente entre SH y riesgo general de infección peritoneal.
ConclusiónLa sobrehidratación persistente asocia un riesgo significativo de infección peritoneal por patógenos entéricos, en pacientes tratados con DP.
Persistent or recurrent volume overload (VO) is very frequent in dialysis, affecting incident and prevalent patients, treated with either haemodialysis or with peritoneal dialysis (PD).1 More than half of patients in chronic PD suffer some degree of VO, often from the very onset of treatment.2–4 This complication is considered a significant source of cardiovascular risk5–7 and, in general, of poor prognosis for these patients.8–11
The correct diagnosis and effective treatment of hypervolemia are relevant objectives, within the general management of patients with PD. However, detecting and quantifying VO poses important challenges in daily clinical practice. The diagnosis of VO is relatively easy only when it is major and it is associated to clinical signs (rapid weight gain, peripheral oedema, progressive hypertension, left ventricular failure). Early diagnosis and management of this complication require more precise instruments.12 In recent years, the assessment of body composition using multi-frequency bioimpedance analysis (BIA) has achieved a remarkable acceptance as a method of assessing body composition and hydration status, due to its simplicity, minimal side effects and reasonable accuracy.13,14 BIA devices measure resistance and body reactance to electric currents, and use these estimates to calculate total body water (TBW) volumes and their intracellular (ICW) and extracellular (ECW) fractions. These calculations can be used to detect and quantify VO.15 A large majority of clinical studies with estimation of volemia in patients with end-stage renal disease published in the last decade have been based on BIA.1,2,4,8,16,17
Peritoneal infection is one of the most feared complications of PD, with significant effects on mortality and increased rates of technique failure.18–21 In recent decades, the application of different preventive measures has progressively reduced the overall incidence of this complication. Unfortunately, the aforementioned measures have had a very uneven impact on the incidence of peritoneal infections of different aetiologies. In particular, infections by micro-organisms of enteric origin have maintained a significant incidence.22 The design of strategies aimed at reducing the incidence of enteric peritonitis demands an adequate knowledge of its pathogenic mechanisms and precipitating factors.18 Of particular interest is the possibility that persistent oedema of the intestinal wall can allow the transmigration of toxins and bacteria through it, with systemic consequences in different clinical contexts.23 This idea has fuelled the hypothesis that VO could favour the development of peritonitis of enteric origin in patients treated with PD.
We present the results of a prospective study, aimed at investigating the possible association between persistent VO (estimated by serial analysis of body composition by BIA) and the risk of peritoneal infection in patients on PD, with a particular emphasis on the risk of infection due to germs of enteric origin.
Population and methodGeneral designFollowing a prospective and observational design, we monitored the state of extracellular volume and the clinical evolution of incident patients treated with PD in our centre during the period 2011-16. The study variables were the essential parameters of body composition obtained through serial analysis performed with BIA, with particular emphasis on the markers of VO (overhydration [OH], estimated both in absolute terms and in the form of ratios or fractions ECW/ICW, OH/ECW and ECW/TBW) (main study variables). The main outcome variable was survival at the first episode of peritoneal infection by enteric germs.
The study protocol was approved by the ethics committee of our centre, and oral informed consent was obtained from the participants. The study was adapted to the general principles of the Declaration of Helsinki.
Study populationAll patients over 18 years of age who experienced PD during the recruitment period were considered eligible. We excluded from the analysis those who did not have at least one study of body composition during the first 3 months of treatment with PD, as well as those who did not have a minimum exposure period of 3 months at risk of peritoneal infection. No patient refused their consent to participate in the study.
Study variablesBody composition studies were conducted using a commercially available BIA device (BCM, Fresenius, RFA), at baseline and every 3 months, until the end of the first year. Estimates made under conditions of instability were not taken into account (e.g., during cardiovascular events). We recorded the following parameters for the study: lean mass index (LMI) (lean mass/height2), fat mass index (FMI) (fat mass/height2) and OH, defined by the difference between the expected ECW under normal physiological conditions and that estimated by BIA (absolute OH) and through the ratios ECW/ICW, OH/ECW and ECW/TBW (relative OH). For the analyses, the mean values of the estimates obtained during the first year on PD, or of all available estimates, were used in the case of patients with less than one year of follow-up.
The outcome variables were survival at the first episode of enteric peritonitis (main) or peritonitis of any aetiology (secondary). Peritoneal infection was defined according to criteria of the International Peritoneal Dialysis Society.24 Enteric peritonitis were defined as all those with at least one isolation of Enterobacteriaceae and/or Enterococcus spp and/or intestinal anaerobes, both in mono- and polymicrobial context.
The main clinical and demographic variables with potential association to clinical outcomes were recorded, including the risk of peritoneal infection (Table 1).
Study population. Main demographic and laboratory variables at the start of PD.
| No. | 139 |
|---|---|
| Age; years | 61.1 ± 14.4 |
| Gender (male/female); % | 93/46 (66.9/33.1) |
| PD category (CAPD [continuous ambulatory peritoneal dialysis]/automatic); % | 118/21 (84.9/15.1) |
| Diabetes; % | 58 (41.8) |
| Comorbidities; no. | 1.39 ± 1.51 |
| Prior immunosuppression; % | 20 (14.4) |
| Glomerular filtration (mean renal clearance); ml/min | 8.6 ± 4.0 |
| D/P240′ creatinine, baseline PBT | 0.71 ± 0.09 |
| Ultrafiltration at 240′, baseline PBT; ml | 525 ± 270 |
| Body mass index; kg/m2 | 27.4 ± 4.2 |
| Haemoglobin; g/dl | 10.9 ± 1.3 |
| Plasma albumin; g/l | 37.5 ± 5.7 |
| C-reactive protein; mg/dl | 0.42 (0.01–15.86) |
| Follow-up; months | 24.0 ± 17.2 |
PD: peritoneal dialysis; PBT: peritoneal balance test.
The figures express means ± standard deviation for numeric variables (except C-reactive protein, presented as median with amplitude), and n (%) for categorical variables.
The study variables were categorised by tertiles, for better understanding in clinical terms. The basic univariate comparisons were performed using the Student's t tests, ANOVA, distribution χ2 and Spearman's correlation coefficient. Univariate survival analyses were performed using the Kaplan-Meier method (log-rank test). The multivariate analysis was carried out using Cox models in steps, controlling for other demographic, clinical or laboratory variables with recognised impact on the outcome variables (age, presence of diabetes, glomerular filtration rate, albumin, C-reactive protein), regardless of the significant association observed in our population. SPSS® v.19.0 software was used for data analysis.
ResultsOverview139 patients were included in the final analysis. The characteristics of the study population at the beginning of PD are presented in Table 1. The most frequent renal diseases were diabetic nephropathy (n = 45) and chronic glomerular nephropathies (n = 20). According to the study protocol, all patients had at least one body composition analysis, 87.1 % had at least 2 analyses, 67.4 % at least 3 analyses and 57.6 % 4 analyses, during the first year on PD. Forty patients (28.8 %) did not reach one year of follow-up, with inconsistencies in the values obtained, protocol violations and clinical instability at the time of the scheduled estimate being more sporadic causes for non-availability of 4 body composition values. The body composition parameters are shown in Table 2.
Studies of body composition.
| Baseline | Mean of first year | |
|---|---|---|
| Body weight; kg | 74.7 ± 12.9 | 75.6 ± 13.1 |
| OH; l | 1.17 ± 1.79 (–2.6/8.5) | 1.12 ± 1.56 (–2.7/5.4) |
| Extracellular water; l | 16.8 ± 3.1 | 16.9 ± 3.0 |
| Intracellular water; l | 18.2 ± 3.9 | 18.3 ± 3.8 |
| ECW/ICW ratio | 0.94 ± 0.14 | 0.94 ± 0.13 |
| OH/ECW ratio | 0.07 ± 0.12 | 0.07 ± 0.16 |
| ECW/TBW ratio | 0.48 ± 0.04 | 0.48 ± 0.04 |
| Fat mass index; kg/m2 | 10.0 ± 3.8 | 10.3 ± 3.6 |
| Lean mass index; kg/m2 | 13.1 ± 3.0 | 13.1 ± 2.8 |
TBW: total body water; ECW: extracellular water; ICW: intracellular water; OH: overhydration.
The figures express means ± standard deviation (breadth). "Baseline" refers to the first BIA performed after the start of PD. "Mean" indicates the mean of the estimates during the first year (or until the end of follow-up if it is less than one year).
The serial estimates of body composition were very consistent regarding LMI (always r > 0.85; p < 0.0005, Spearman), FMI (always r > 0.75; p < 0.0005) and, to a lesser extent, OH (r = 0.57 first versus second, r = 0.44 first versus third, p < 0/0005), and ECW/ICW (r = 0.63 first versus second, r = 0.66 first versus third, p < 0.0005), OH/ECW (r = 0.65 first versus second, r = 0.58 first versus third, p < 0.0005) and ECW/TBW (r = 0.63 first versus second, r = 0.61 first versus third, p < 0.0005).
Sixty-three patients (45.3 %) suffered at least one episode of peritoneal infection, an average of 15.2 ± 13.5 months after the start of PD, for a total of 134 episodes. The main aetiological agents were gram-positive germs (n = 79), including 51 episodes from Streptococcus spp. There were also 15 episodes of infection by enteric and non-enteric gram-negative germs, and 21 polymicrobial infections while, in 13 cases, bacteriological studies were negative. Seventeen patients (12.2 %) experienced at least one mono- or polymicrobial infection with isolation of enteric micro-organisms, for a total of 25 episodes (cumulative incidence of one episode every 133.4 patients/months). At the end of follow-up, 9 patients had died, and 3 had been transferred to haemodialysis due to severe or recurrent peritonitis.
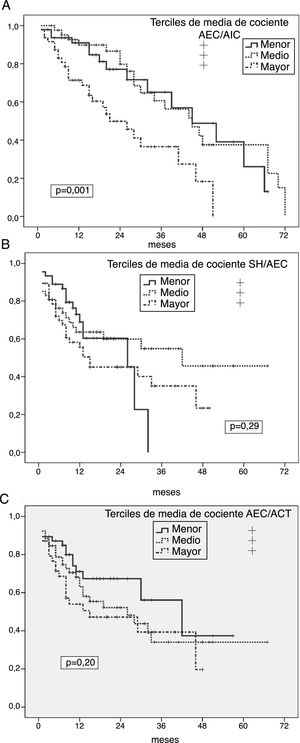
Univariate predictors of peritoneal infectionGeneral risk of peritonitisKaplan-Meier analyses showed an increased risk of peritonitis in the highest thirds of ICW/ECW ratio (p = 0.001) (Fig. 1 A), and significantly lower in the lower tertile of FMI (p = 0.024), while absolute OH, OH/ECW (Fig. 1B), ECW/TBW (Fig. 1C) or LMI showed no correlation with this outcome variable. However, patients who presented at least one episode of peritonitis showed higher degrees of absolute OH (1.46 ± 1.64 versus 0.92 ± 1.87 l; p = 0.059), ECW/ICW ratio (0.98 ± 0.13 versus 0.90 ± 0.13; p = 0.002) and ECW/TBW ratio (0.49 ± 0.03 versus 0.47 ± 0.03, p = 0.004) (p = 0.088 for OH/ECW) at the start of PD than those who did not present this complication.
A) Probability of peritoneal infection (any aetiology) in relation to the average estimates of the ECW/ICW ratio during the first year on PD. B) Probability of peritoneal infection (any aetiology) in relation to the average estimates of the OH/ECW ratio during the first year on PD. C) Probability of peritoneal infection (any aetiology) in relation to the average estimates of the ECW/TBW ratio during the first year on PD.
In relation to other variables, the overall risk of peritonitis was higher in diabetics (p = 0.001) and patients with lower glomerular filtration at the start of PD (p = 0.042), with non-significant trends in cases of patients with depressive disorders (p = 0.072) (all other variables in Table 1 not significant NS).
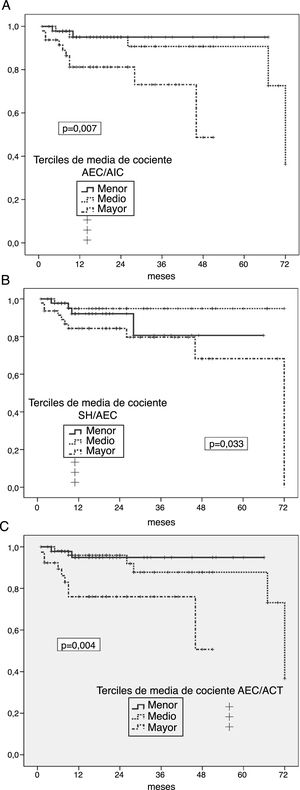
Risk of enteric germ peritonitisUnivariate analyses showed a significantly increased risk of enteric peritonitis in patients in the highest third of ECW/ICW ratio (p = 0.007) (Fig. 2 A), OH/ECW (Fig. 2B) and ECW/TBW (Fig. 2C). In the case of absolute OH, only one trend in the same direction was observed (p = 0.096). On the contrary, the highest third of LMI associated a tendency to lower risk of this complication (p = 0.092) (FMI NS). Patients who suffered some episode of enteric peritonitis had higher degrees of absolute OH (1.92 ± 1.83 versus 1.03 ± 1.73 l; p = 0.059), ECW/ICW ratio (1.02 ± 0.17 versus 0.92 ± 0.13; p = 0.012) OH/ECW ratio (0.12 ± 0.11 versus 0.05 ± 0.09; p = 0.016), and ECW/TBW ratio (0.51 ± 0.04 versus 0.46 ± 0.04; p = 0.010) at the start of PD than those who did not present this complication.
A) Probability of enteric peritoneal infection in relation to the average estimates of the ECW/ICW ratio during the first year on PD. B) Probability of enteric peritoneal infection in relation to the average estimates of the OH/ECW ratio during the first year on PD. C) Probability of enteric peritoneal infection in relation to the average estimates of the ECW/TBW ratio during the first year on PD.
High levels of C-reactive protein (p = 0.056) and lower levels of plasma albumin (p = 0.11) at the start of PD were the only variables that showed a trend (not significant) to univariate association with the risk of enteric peritonitis during follow-up.
Multivariate analysisThe results of the multivariate analysis are presented in Table 3. Apart from an NS trend to lower risk in patients with lower levels of FMI, no body composition parameter showed an association with the probability of suffering from a peritoneal infection (any aetiology). On the contrary, our analyses revealed a consistent correlation between the persistent presence of VO (estimated from the ICW/ECW, OH/ECW and ECW/TBW ratios) and the subsequent risk of suffering at least one episode of enteric germ peritonitis. It should be noted that, in the case of the OH/ECW ratio, the risk ratio between the highest and lowest thirds did not reach statistical significance (Table 3), but the comparison between highest and middle thirds (RR: 3.67; 95 % CI: 1.02/13.51; p = 0.044).
Risk of peritoneal infection in relation to body composition analyses. Multivariate study.
| [0,2–4]Peritonitis (all) | [0,5–7]Peritonitis (enteric) | |||||
|---|---|---|---|---|---|---|
| RR | 95 % CI | p value | RR | 95 % CI | p value | |
| [0,1–7]Mean OH | ||||||
| Middle tertile | 0.80 | 0.41/1.56 | 0.51 | 0.84 | 0.87/4.22 | 0.84 |
| High tertile | 1.11 | 0.60/2.06 | 0.73 | 2.17 | 0.75/7.55 | 0.25 |
| [0,1–7] | ||||||
| [0,1–7]Mean ECW/ICW | ||||||
| Middle tertile | 1.30 | 0.64/2.64 | 0.47 | 1.00 | 0.36/5.14 | 0.98 |
| High tertile | 1.52 | 0.76/3.03 | 0.23 | 3.48 | 1.03/14.59 | 0.046 |
| [0,1–7] | ||||||
| [0,1–7]Mean OH/ECW | ||||||
| Middle tertile | 0.98 | 0.50/1.92 | 0.95 | 0.69 | 0.29/2.45 | 0.28 |
| High tertile | 1.49 | 0.80/2.80 | 0.21 | 2.31 | 0.98/6.56 | 0.061 |
| [0,1–7] | ||||||
| [0,1–7]Mean ECW/TBW | ||||||
| Middle tertile | 1.45 | 0.78/2.75 | 0.25 | 1.50 | 0.67/8.23 | 0.33 |
| High tertile | 1.80 | 0.93/3.49 | 0.086 | 6.33 | 1.37/19.37 | 0.011 |
| [0,1–7] | ||||||
| [0,1–7]Mean FMI | ||||||
| Middle tertile | 2.05 | 1.00/4.21 | 0.049 | 0.61 | 0.17/2.15 | 0.44 |
| High tertile | 1.88 | 0.98/3.56 | 0.061 | 0.67 | 0.19/2.35 | 0.53 |
| [0,1–7] | ||||||
| [0,1–7]Mean LMI | ||||||
| Middle tertile | 0.89 | 0.48/1.65 | 0.72 | 1.52 | 0.55/4.78 | 0.38 |
| High tertile | 0.88 | 0.47/1.67 | 0.70 | 0.88 | 0.29/1.76 | 0.75 |
TBW: total body water; ECW: extracellular water; ICW: intracellular water; CI: confidence interval; FMI: fat mass index; LMI: lean mass index; RR: relative risk; OH: overhydration.
Reference low tertile.
The figures express adjusted coefficients. Main control variables: glomerular filtration rate, diabetes (risk of peritonitis of any aetiology), plasma albumin and C-reactive protein (risk of peritonitis, global or enteric).
Both peritoneal and VO infections have a known negative effect on the evolution of patients on PD. Traditionally, it has been considered that these complications compromised patient survival and PD technique by differentiated mechanisms, but more recently, it has been suggested that persistent VO may be a modifiable risk factor for PD peritonitis. Thus, in a post hoc analysis of a cross-sectional study, aimed at investigating the prevalence and risk factors for OH in 307 patients on PD in China, Guo et al.5 observed that patients with higher levels of ECW/TBW ratio had worse results in terms of patient survival and technique, and apparently increased risks of peritonitis and cardiovascular events. On the other hand, Santhakumaran et al.25 presented the results of a retrospective study of patients with at least 2 body composition analyses, and compared the rates of peritoneal infection of patients categorised according to the median OH/ECW ratio, used as an estimator of OH. The univariate analysis showed a clear trend towards increased risk of enteric germ peritonitis in overhydrated patients, although this association was not maintained after adjusting the coefficients for plasma albumin levels and glomerular filtration.
Isolation of micro-organisms of enteric origin during a peritoneal infection in PD is usually associated with aggressive clinical presentations and poor overall prognosis, including substantial mortality and technique failure rates.18,26,27 Despite continued efforts in recent decades, the overall incidence of these infections has not improved significantly. A better knowledge of its pathogenesis could be of great help, for this purpose, but the information available is limited and often controversial. The presence of pre-existing (e.g., diverticulosis) or recently started (e.g., intestinal ischemia or acute cholecystitis) gaps in the continuity of abdominal antimicrobial barriers represents an obvious source for such infections. Many other factors have been linked to an increased risk of enteric peritonitis on PD, including diabetes, general comorbidity, malnutrition, inflammation, constipation, diarrhoea, laxative abuse, persistent hypokalaemia, broad-spectrum antibiotic therapy or treatments with gastric acid secretion inhibitors.28 On the other hand, and in a manner comparable to other forms of peritonitis, the PD technique itself may contribute to the risk of enteric infections. For example, hypertonic solutions, particularly if they are rich in glucose degradation products, can generate local inflammation and affect peritoneal defence mechanisms.29 In addition, the in situ presence of the peritoneal catheter and the dialysis mechanics itself (including recurrent renewal of the peritoneal fluid) may contribute to a defective immune response in these patients.26
To our knowledge, this is the first prospective study that demonstrates an association between persistent OH and the risk of peritoneal infection by enteric germs. However, the explanation for this association is not entirely clear. It has been suggested that persistent oedema of the intestinal wall may favour microbial and bacterial endotoxin transmigration, in some cases leading to systemic infections, in different clinical contexts. Patients with congestive heart failure have intestinal microcirculation disorders, which appear to contribute to the inflammatory state observed in these cases.23 The potential analogy with patients on PD with persistent OH is very attractive. Previous studies have shown an association between persistent OH and inflammatory markers in these patients,30 and this study showed a trend toward association between baseline levels of C-reactive protein and the subsequent risk of enteric peritonitis. However, these are clearly speculative hypotheses, because the association between VO, intestinal wall oedema and risk of local or systemic infection seems inconsistent. In addition, and from an opposite perspective, there is no evidence that patients treated with peritoneal ultrafiltration due to refractory heart failure, or patients on PD suffering from anasarca phases present a particular risk of infection by enteric germs. Finally, alternative explanations must be considered. For example, O-H may be a mere marker of subjects with a greater intrinsic risk of peritonitis on PD (elderly, diabetic, malnourished, hypoalbuminaemic or with cardiovascular disease) although, in this case, a globally increased risk of peritoneal infection would be expected, not specifically restricted to infections by intestinal microorganisms.
This study has significant limitations, including its single-centre design and a sample of limited size, which may have affected the consistency of some results. For example, the ECW/ICW and ECW/TBW ratios, but not the absolute OH, showed association with the main outcome variable, while the OH/ECW ratio showed a clear trend that, however, was not fully consistent. Our multivariate model considered the main confounding variables with a recognized association with the risk of peritonitis in PD, but limited knowledge of the pathogenic factors for enteric peritonitis (including the hidden presence of abdominal disease) adds uncertainty to the reliability of the results.
In summary, persistent volume overload associates a significant risk of peritoneal infection by micro-organisms of enteric origin in patients treated with PD. On the contrary, the overall risk of peritoneal infection does not appear to be increased, in this context. The prevention and early and effective management of volume overload should be part of any strategy aimed at reducing the incidence of enteric peritonitis in these patients.
Conflict of interestsThe authors declare that they have no conflicts of interest.
Please cite this article as: Carvalho Fiel D et al. La sobrehidratación persistente asocia un riesgo significativo de infección peritoneal por gérmenes entéricos en pacientes tratados con Diálisis Peritoneal. Nefrologia. 2019;39:638–645.