Rotational coronary angiography (RCA) requires less contrast to be administered and can prevent the onset of contrast-induced nephropathy (CIN) during invasive coronary procedures. The aim of the study is to evaluate the impact of RCA on CIN (increase in serum creatinine ≥0.5mg/dL or ≥25%) after an acute coronary syndrome.
MethodsFrom April to September 2016, patients suffering acute coronary syndromes who underwent diagnostic coronary angiography, with the possibility of ad hoc coronary angioplasty, were prospectively enrolled. At the operator's discretion, patients underwent RCA or conventional coronary angiography (CCA). CIN (primary endpoint), as well as analytical, angiographic and clinical endpoints, were compared between groups.
ResultsOf the 235 patients enrolled, 116 patients received RCA and 119 patients received CCA. The RCA group was composed of older patients (64.0±11.8 years vs. 59.7±12.1 years; p=0.006), a higher proportion of women (44.8 vs. 17.6%; p<0.001), patients with a lower estimated glomerular filtration rate (76±25 vs. 86±27ml/min/1.73m2; p=0.001), and patients who underwent fewer coronary angioplasties (p<0.001) compared with the CCA group. Furthermore, the RCA group, received less contrast (113±92 vs. 169±103ml; p<0.001), including in diagnostic procedures (54±24 vs. 85±56ml; p<0.001) and diagnostic-therapeutic procedures (174±64 vs. 205±98ml; p=0.049) compared with the CCA group. The RCA group presented less CIN (4.3 vs. 22.7%; p<0.001) compared to the CCA group, and this finding was maintained in the regression analysis (Adjusted relative risk: 0.868; 95% CI: 0.794–0.949; p=0.002). There were no differences in clinical endpoints between the groups.
ConclusionsRCA was associated with lower administration of contrast during invasive coronary procedures in acute coronary syndrome patients, resulting in lower incidence of CIN, in comparison with CCA.
La angiografía coronaria rotacional (ACR) permite reducir la cantidad de contraste administrado y puede prevenir el desarrollo de nefropatía inducida por contraste (NIC) durante los procedimientos coronarios invasivos. El objetivo del estudio es evaluar el impacto de la ACR en la aparición de NIC (aumento de creatinina ≥0,5mg/dL o ≥25%) tras un síndrome coronario agudo.
MétodosDe abril a septiembre de 2016 se seleccionaron prospectivamente pacientes con síndrome coronario agudo remitidos para coronariografía diagnóstica con posibilidad de angioplastia ad hoc, que fueron estudiados con ACR o angiografía coronaria convencional (ACC) según criterio del operador. Se compararon la NIC (variable de valoración primaria), variables analíticas, angiográficas y clínicas.
ResultadosDe 235 pacientes reclutados, 116 pacientes fueron estudiados con ACR y 119 pacientes con ACC. El grupo de ACR presentaba mayor edad (64,0±11,8 vs. 59,7±12,1 años; p=0,006), más mujeres (44,8 vs. 17,6%; p<0,001) y peor filtrado glomerular estimado (76±25 vs. 86±27mL/min/1,73m2; p=0,001), con menos angioplastias (p<0,001). Asimismo, el grupo de ACR recibió menos contraste (113±92 vs. 169±103mL; p<0,001), diferencias que se mantuvieron en los procedimientos diagnósticos (54±24 vs. 85±56mL; p<0,001) y diagnóstico-terapéuticos (174±64 vs. 205±98mL; p=0,049). El grupo de ACR presentó menos NIC (4,3 vs. 22,7%; p<0,001): en el análisis de regresión se objetivó que continuaba relacionándose con menor desarrollo de NIC (riesgo relativo ajustado: 0,868; IC 95%: 0,794-0,949; p=0,002). No hubo diferencias en las variables clínicas.
ConclusionesLa ACR se asoció con menor administración de contraste durante procedimientos coronarios invasivos tras un síndrome coronario agudo, lo que resultó en una menor aparición de NIC.
Contrast-induced nephropathy (CIN), occurs in 1–33% of patients undergoing invasive coronary angiography procedures. It is one of the most common causes of acute renal failure in cardiac patients, especially in cases of acute coronary syndrome (ACS).1–4 The development of CIN after an invasive coronary procedure is associated with prolonged hospitalization, a marked increase in morbidity and mortality, and an increase in health costs.2,5
Published clinical trials have not focused on specific techniques aiming to prevent CIN, but rather on hydration strategies or the administration of drugs. It should be noted that, apart from hydration, most of the previous studies on the prevention of CIN have had neutral effects, and in the case of N-acetylcysteine administration there have been contradictory results.6–15
Coronary angiography is the gold standard method to evaluate of coronary arteries. Conventional coronary angiography (CCA) requires several angiographic projections and requires, in multiple cases, additional projections to obtain an adequate evaluation of the coronary arteries.16 The volume of iodinated contrast used in invasive coronary procedures is closely related to the occurrence of CIN. Now it is available the rotational coronary angiography (RCA) of double axis (craniocaudal and left-right) that due to its rapid rotational movement in both axes it is possible to perform a complete study with a single contrast injection for each coronary artery.17–19 However, despite its potential benefits, RCA is not routinely used in most centers given the lack of evidence that its performance is associated with improvement of clinical assessment variables such as CIN. For this reason, clinical practice guidelines do not mention this technique to prevent CIN.20
Therefore, the objective of our study is to compare two angiographic techniques: the RCA and the CCA, with the aim of determining whether the reduction in the volume of iodinated contrast when performing the RCA allows to reduce CIN in patients with ACS treated with invasive coronary procedures.
MethodsPatients and study designThis work is an observational and prospective study that evaluates the development of CIN in 2 cohorts of consecutive patients with ACS (unstable angina or acute myocardial infarction [AMI])4 referred for invasive coronary procedures in 2 institutions: the University Hospital of Nuestra Señora de Candelaria (Santa Cruz de Tenerife, Spain) and the University Hospital of the Canary Islands (San Cristóbal de La Laguna, Tenerife, Spain). The type of angiography was performed according to the operator's criteria. Patients included were those with consecutive ACS with indication of invasive coronary angiography who were not on renal replacement therapy and who did not present an AMI with ST segment elevation of less than 12h of evolution since the performance of an emergent coronary angiography does not allow a procedure regulated on multiple occasions.
The Ethical and Clinical Research Committee of the University Hospital Nuestra Señora de Candelaria and the University Hospital of the Canary Islands approved the protocol of this study, since it complies with current ethical and legal regulations.
Inclusion criteria- -
Indication of invasive coronary angiography by ACS with or without percutaneous coronary intervention.
- -
Informed consent.
- -
Patients <18 years.
- -
Previous renal replacement therapy.
- -
Women with possibilities of being pregnant.
- -
Allergy to iodinated contrast previously known, that cannot receive premedication.
- -
Exposure to iodinated contrast in the previous 10 days.
- -
Previous myocardial revascularization surgery.
- -
AMI with ST-segment elevation of <12h of evolution.
- -
Cardiogenic shock.
- -
Inability to understand the nature of the study or medical or social disability that may interfere with the collection of data or appropriate follow up.
- -
Inclusion in other clinical trials or registries.
- -
CIN: increase in creatinine ≥0.5mg/dL or ≥25% from the baseline to 48 and 72h after the procedure.21
- -
CIN using the criteria of acute renal failure induced by iodinated contrast (AKI-IC): increase in serum creatinine >50% or >0.3mg/dL from the baseline to 48 and 72h after the procedure.22
- -
CIN using estimated glomerular filtration (eGFR): decrease in eGFR ≥25% from the baseline to 48 and 72h after the procedure.
- -
Combined clinical assessment that included the following: global mortality, new infarction, cerebrovascular accident and need of dialysis during hospitalization at 30 days. Each of these conditions were also assessed separately during admission and at 30 days. Mortality from all causes included cardiac death, vascular death and non-cardiovascular death.24 A new myocardial infarction was defined as a myocardial infarction occurring after the invasive coronary procedure and matching the third universal definition of myocardial infarction (type 1).25 The cerebrovascular accident was defined as an acute episode of focal dysfunction cerebral or monocular, transient or persistent in time, caused by thrombosis or arterial embolism. The presence of alterations in specific imaging techniques was not required for such a diagnosis.26 The need for dialysis was defined as the medical indication of renal replacement therapy with hemodialysis due to severe dererioration of renal function.27
- -
The new hospitalization was defined as a new hospital admission of more than 24h in duration for the treatment or diagnosis of any medical condition after performing the invasive coronary procedure.24
- -
Radiation dermatitis was defined as any of the following cutaneous alterations: erythema, edema, bleeding, ulceration or skin necrosis, in the areas of incidence of the X-ray beam that occurred after performing an invasive coronary procedure in the absence of an alternative cause.28
Patients received treatment for ACS according to standard clinical practice. If they had a baseline creatinine ≥1.59mg/dL, patients received normal saline (0.9% sodium chloride) at a rate of 1mL/kg/h during the 12h before and 24h after the procedure, unless there were contraindications.20
The CCA was performed according to the following recommendations: a minimum of 6 projections for the left coronary artery and a minimum of 3 projections for the right coronary artery. However, the final number of projections for a correct assessment of the coronary arteries or planning of percutaneous coronary intervention was left to the discretion of the operator. The volume of contrast used was from 8 to 4mL/s for the left coronary artery and from 5 to 3mL/s for the right coronary artery. The RCA was performed according to recommendations. In order to obtain optimal quality images by means of RCA, it was decided to perform a 5.8s rotation in the left coronary artery and the volume injected was 14–2.5mL/s; for the right coronary artery, it was performed a 4s rotation and an injection of 10–2mL/s.19
The procedure of percutaneous coronary intervention ad hoc was left to the discretion of the medical team and it was performed according to the usual clinical practice. The procedure of ventriculography was also done at the discretion of the operator. If it was considered adequate, it was injection of 45 to 15mL/s or from 36 to 12mL/s depending on the degree of renal dysfunction. A third-generation contrast agent was used: iomeron 350 (Iomeprol, Bracco Corporate, Milan, Italy) and, to standardize the administration of contrast, it was used the ACIST CVi® robotic contrast injector (ACIST Medical Systems, Eden Prairie, MN, US). The choice of coronary catheters was left to the discretion of the operator. The acquisition of the images was done according to the recommended practice for the RCA.19
Data collection and follow upBaseline samples were obtained to measure baseline serum creatinine levels (with the Jaffé method) and to calculate eGFR using the CK-EPI formula. Samples were also obtained after 48–72h of the procedure to evaluate changes in renal function.29,30 In the case of patients who were discharged before 48h after the procedure, the patient came to the outpatient clinic for blood sampling in the period of time specified in the study. Likewise, we collected data related to the procedure, clinical variables, angiographic (location and number of coronary vessels with stenosis >50%), radiation, analytical data and also the length of hospital stay (days).
Patients were visited at day 30 to evaluate clinical variables. The information was collected prospectively and entered into a specifically designed computerized database.
Sample size calculationSample size was calculated using GRANMO software (Institut Municipal d’Investigació Mèdica, Barcelona, Spain). The sample size was calculated to demonstrate a reduction from 25% in the cohort of patients analyzed with CCA to at least 10% in the cohort of patients analyzed with RCA (relative risk ACR/ACC≤0.4), with a ratio of patients between the groups of 1:1 and a proportion of losses in the follow-up of 3%. Using a χ2 test for 2×2 tables, with an alpha risk of 0.05 and a beta risk of less than 0.2 (statistical power of 80%) in a bilateral contrast, the calculated sample size was 116 subjects per arm to detect statistical difference between 2 proportions (232 patients in total).31
Statistic analysisStatistical analysis was performed with SPSS Statistics 24.0 software (SPSS Inc., Chicago, IL, United States). Two tails p<0.05 were considered statistically significant. The normality of the continuous variables was explored by the Kolmogorov–Smirnov test. The categorical variables were expressed as percentage. The categorical variables were compared using the Fisher exact test or the chi-square test, as appropriate. Variables with values with a normal distribution were expressed as mean (standard deviation), and were compared using t tests for 2 samples; the continuous variables without a normal distribution are expressed as median (interquartile range) and were analyzed using the Wilcoxon nonparametric test (rank-sum).
Binary logistic regression models were done to establish the independent predictors of CIN. A univariate exploratory analysis was performed, introducing in the model the covariates that had a p<0.10. Due to the small number of events in the primary end point (CIN), only 2 variables of important clinical relevance were added to the model: the presence of previous renal dysfunction (eGFR<60mL/min/1.73m2) and the execution of percutaneous coronary interventionism.2,3 The final model included the following variables: RCA, diabetes mellitus, contrast volume ≥300mL, dose×area product ≥50Gycm2, percutaneous coronary intervention and eGFR <60mL/min/1.73m2. The results are reported as odds ratio (OR) with a 95% confidence interval (95% CI).
Finally, to evaluate the association between the RCA (exposure variable) and the CIN (primary endpoint variable), a Poisson regression was performed introducing the independent predictor variables of CIN in the model. Results were reported as a relative risk (RR) adjusted with a CI of 95%.
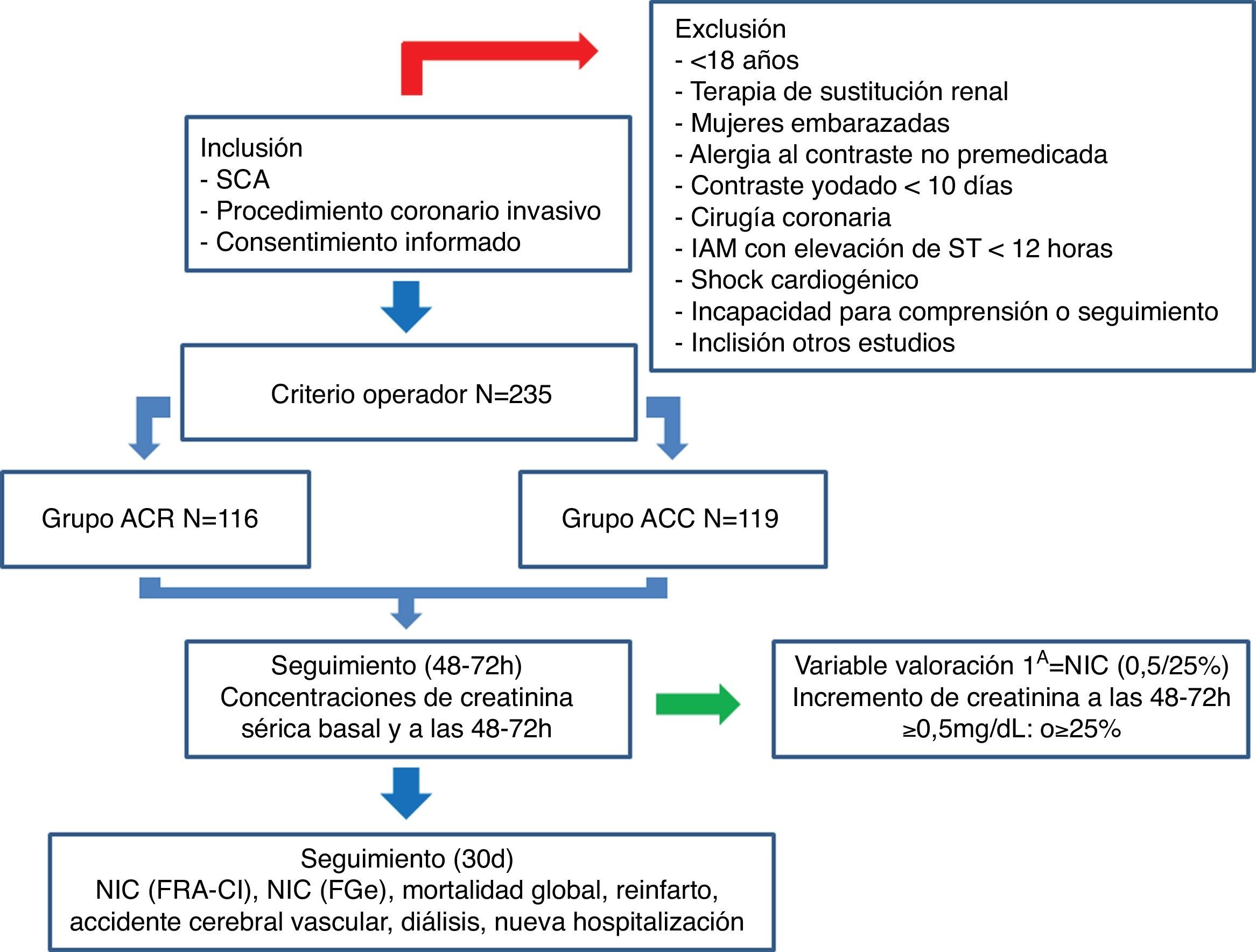
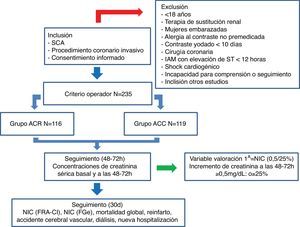
ResultsFrom April to September 2016, a total of 235 patients with ACS had coronary angiography performed and were included in the analysis. Of these, 116 patients were evaluated with RCA (49.4%) and 119 with CCA (50.4%). The flow diagram of the study is represented in Fig. 1.
Flow diagram of the CINERAMA study. CCA: conventional coronary arteriography; RCA: rotational coronary arteriography; ACS: acute coronary syndrome; eGFR: estimated glomerular filtration rate; AKI-CI: contrast-induced acute kidney injury; AMI: acute myocardial infarction; CIN: contrast-induced nephropathy.
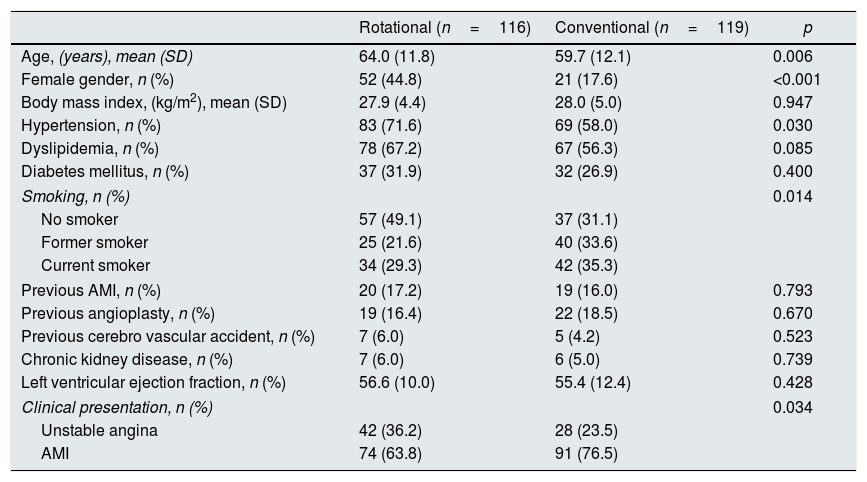
The baseline characteristics of both groups are presented in Table 1. Patients from the RCA group were older (64.0±11.8 vs. 59.7±12.1 years, p<0.001) with a higher percentage of women (44.8% vs. 17.6%, p<0.001) and higher percentage of hypertension (71.6% vs. 58.0%, p=0.03). Likewise, the RCA group had a lower proportion of smokers (p=0.014) and less percent of AMI as the clinical presentation (63.8 vs. 76.5%, p=0.034).
Baseline clinical characteristics.
| Rotational (n=116) | Conventional (n=119) | p | |
|---|---|---|---|
| Age, (years), mean (SD) | 64.0 (11.8) | 59.7 (12.1) | 0.006 |
| Female gender, n (%) | 52 (44.8) | 21 (17.6) | <0.001 |
| Body mass index, (kg/m2), mean (SD) | 27.9 (4.4) | 28.0 (5.0) | 0.947 |
| Hypertension, n (%) | 83 (71.6) | 69 (58.0) | 0.030 |
| Dyslipidemia, n (%) | 78 (67.2) | 67 (56.3) | 0.085 |
| Diabetes mellitus, n (%) | 37 (31.9) | 32 (26.9) | 0.400 |
| Smoking, n (%) | 0.014 | ||
| No smoker | 57 (49.1) | 37 (31.1) | |
| Former smoker | 25 (21.6) | 40 (33.6) | |
| Current smoker | 34 (29.3) | 42 (35.3) | |
| Previous AMI, n (%) | 20 (17.2) | 19 (16.0) | 0.793 |
| Previous angioplasty, n (%) | 19 (16.4) | 22 (18.5) | 0.670 |
| Previous cerebro vascular accident, n (%) | 7 (6.0) | 5 (4.2) | 0.523 |
| Chronic kidney disease, n (%) | 7 (6.0) | 6 (5.0) | 0.739 |
| Left ventricular ejection fraction, n (%) | 56.6 (10.0) | 55.4 (12.4) | 0.428 |
| Clinical presentation, n (%) | 0.034 | ||
| Unstable angina | 42 (36.2) | 28 (23.5) | |
| AMI | 74 (63.8) | 91 (76.5) | |
SD: standard deviation; AMI: acute myocardial infarction.
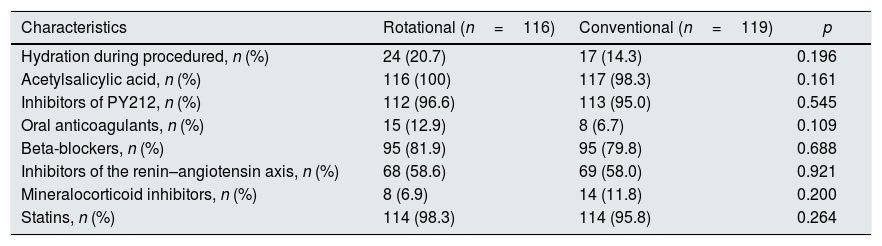
Table 2 shows the medications received. There were no significant differences in medical therapies received with a demonstrated benefit in ACS.
Medical treatments.
| Characteristics | Rotational (n=116) | Conventional (n=119) | p |
|---|---|---|---|
| Hydration during procedured, n (%) | 24 (20.7) | 17 (14.3) | 0.196 |
| Acetylsalicylic acid, n (%) | 116 (100) | 117 (98.3) | 0.161 |
| Inhibitors of PY212, n (%) | 112 (96.6) | 113 (95.0) | 0.545 |
| Oral anticoagulants, n (%) | 15 (12.9) | 8 (6.7) | 0.109 |
| Beta-blockers, n (%) | 95 (81.9) | 95 (79.8) | 0.688 |
| Inhibitors of the renin–angiotensin axis, n (%) | 68 (58.6) | 69 (58.0) | 0.921 |
| Mineralocorticoid inhibitors, n (%) | 8 (6.9) | 14 (11.8) | 0.200 |
| Statins, n (%) | 114 (98.3) | 114 (95.8) | 0.264 |
Inhibitors of PY212: inhibitors of protein Y 212.
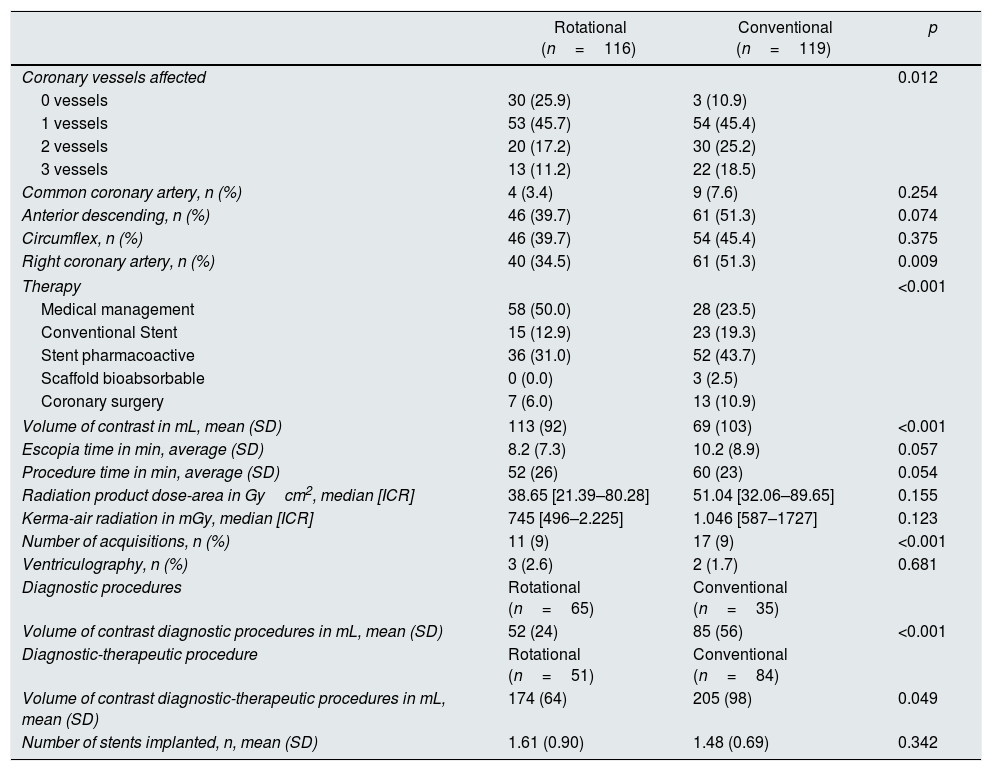
The data comparing the angiographic and radiation parameters between both groups are presented in Table 3.
Angiographic variables and dose of radiation.
| Rotational (n=116) | Conventional (n=119) | p | |
|---|---|---|---|
| Coronary vessels affected | 0.012 | ||
| 0 vessels | 30 (25.9) | 3 (10.9) | |
| 1 vessels | 53 (45.7) | 54 (45.4) | |
| 2 vessels | 20 (17.2) | 30 (25.2) | |
| 3 vessels | 13 (11.2) | 22 (18.5) | |
| Common coronary artery, n (%) | 4 (3.4) | 9 (7.6) | 0.254 |
| Anterior descending, n (%) | 46 (39.7) | 61 (51.3) | 0.074 |
| Circumflex, n (%) | 46 (39.7) | 54 (45.4) | 0.375 |
| Right coronary artery, n (%) | 40 (34.5) | 61 (51.3) | 0.009 |
| Therapy | <0.001 | ||
| Medical management | 58 (50.0) | 28 (23.5) | |
| Conventional Stent | 15 (12.9) | 23 (19.3) | |
| Stent pharmacoactive | 36 (31.0) | 52 (43.7) | |
| Scaffold bioabsorbable | 0 (0.0) | 3 (2.5) | |
| Coronary surgery | 7 (6.0) | 13 (10.9) | |
| Volume of contrast in mL, mean (SD) | 113 (92) | 69 (103) | <0.001 |
| Escopia time in min, average (SD) | 8.2 (7.3) | 10.2 (8.9) | 0.057 |
| Procedure time in min, average (SD) | 52 (26) | 60 (23) | 0.054 |
| Radiation product dose-area in Gycm2, median [ICR] | 38.65 [21.39–80.28] | 51.04 [32.06–89.65] | 0.155 |
| Kerma-air radiation in mGy, median [ICR] | 745 [496–2.225] | 1.046 [587–1727] | 0.123 |
| Number of acquisitions, n (%) | 11 (9) | 17 (9) | <0.001 |
| Ventriculography, n (%) | 3 (2.6) | 2 (1.7) | 0.681 |
| Diagnostic procedures | Rotational (n=65) | Conventional (n=35) | |
| Volume of contrast diagnostic procedures in mL, mean (SD) | 52 (24) | 85 (56) | <0.001 |
| Diagnostic-therapeutic procedure | Rotational (n=51) | Conventional (n=84) | |
| Volume of contrast diagnostic-therapeutic procedures in mL, mean (SD) | 174 (64) | 205 (98) | 0.049 |
| Number of stents implanted, n, mean (SD) | 1.61 (0.90) | 1.48 (0.69) | 0.342 |
SD: standard deviation; ICR: interquartile range.
Patients undergoing RCA had a less extent of coronary disease (p=0.012) and the percent of patients receiving coronary intervention was lower than patients treated with CCA (p<0.001).
Patients treated with RCA received less amount of contrast during the procedures than the CCA patients (113±92 vs. 169±103mL, p<0.001). The amount of contrast was less in RCA than CCA during diagnostic procedure (52±24 vs. 85±56mL, p<0.001) and during the combined diagnostic and therapeutic procedures (174±64 vs. 205±98mL, p=0.049). Likewise, there was also a lower number of acquisitions in the RCA than CCA group (11±9 vs. 17±9, p<0.001).
Of note, there was a tendency to shorter time of escopia with RCA than CCA (8.2±7.3 vs. 10.2±8.9min; p=0.057) and also a shorter duration of the procedure (52±26 vs. 60±23min; p=0.054).
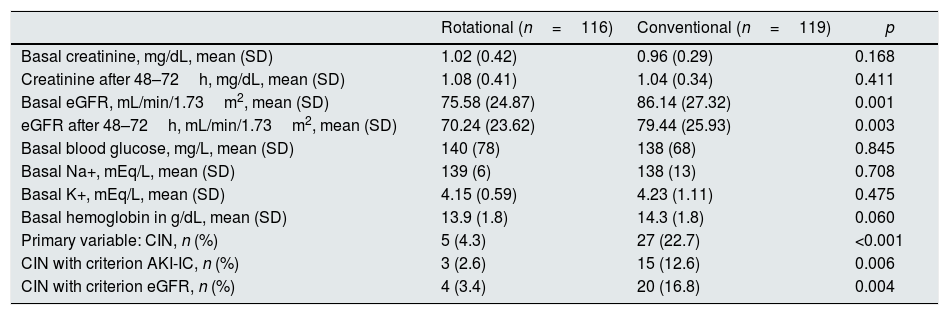
Analytical and CIN variablesTable 4 shows the analytical variables data of and results of CIN (primary assessment variable), also shown is the data on CIN using AKI-IC criteria, and eGFR criteria.
Analytical and CIN variables.
| Rotational (n=116) | Conventional (n=119) | p | |
|---|---|---|---|
| Basal creatinine, mg/dL, mean (SD) | 1.02 (0.42) | 0.96 (0.29) | 0.168 |
| Creatinine after 48–72h, mg/dL, mean (SD) | 1.08 (0.41) | 1.04 (0.34) | 0.411 |
| Basal eGFR, mL/min/1.73m2, mean (SD) | 75.58 (24.87) | 86.14 (27.32) | 0.001 |
| eGFR after 48–72h, mL/min/1.73m2, mean (SD) | 70.24 (23.62) | 79.44 (25.93) | 0.003 |
| Basal blood glucose, mg/L, mean (SD) | 140 (78) | 138 (68) | 0.845 |
| Basal Na+, mEq/L, mean (SD) | 139 (6) | 138 (13) | 0.708 |
| Basal K+, mEq/L, mean (SD) | 4.15 (0.59) | 4.23 (1.11) | 0.475 |
| Basal hemoglobin in g/dL, mean (SD) | 13.9 (1.8) | 14.3 (1.8) | 0.060 |
| Primary variable: CIN, n (%) | 5 (4.3) | 27 (22.7) | <0.001 |
| CIN with criterion AKI-IC, n (%) | 3 (2.6) | 15 (12.6) | 0.006 |
| CIN with criterion eGFR, n (%) | 4 (3.4) | 20 (16.8) | 0.004 |
SD: standard deviation; eGFR: estimated glomerular filtration rate; AKI-CI: acute kidney injury induced by iodinated contrast; K+: potassium; Na+: sodium; CIN: contrast-induced nephropathy.
Baseline eGFR was lower in RCA than CCA group (75.58±24.87 vs. 86.14±27.32mL/min/1.73m2; p=0.001). After the procedure, the eGFR was still lower in RCA than CCA group (70.24±23.62 vs. 79.44±25.23mL/min/1.73m2; p=0.003).
Regarding CIN, the primary end point, it was found that in the RCA cohort CIN was less frequent than CCA (4.3% vs. 22.7%, p<0.001). Likewise, it was also observed that patient from the RCA group had a lower percentage of CIN according to either AKI-IC criteria (2.6% vs. 12.6%, p=0.006) or eGFR criteria (3.4% vs. 16.8%, p=0.004).
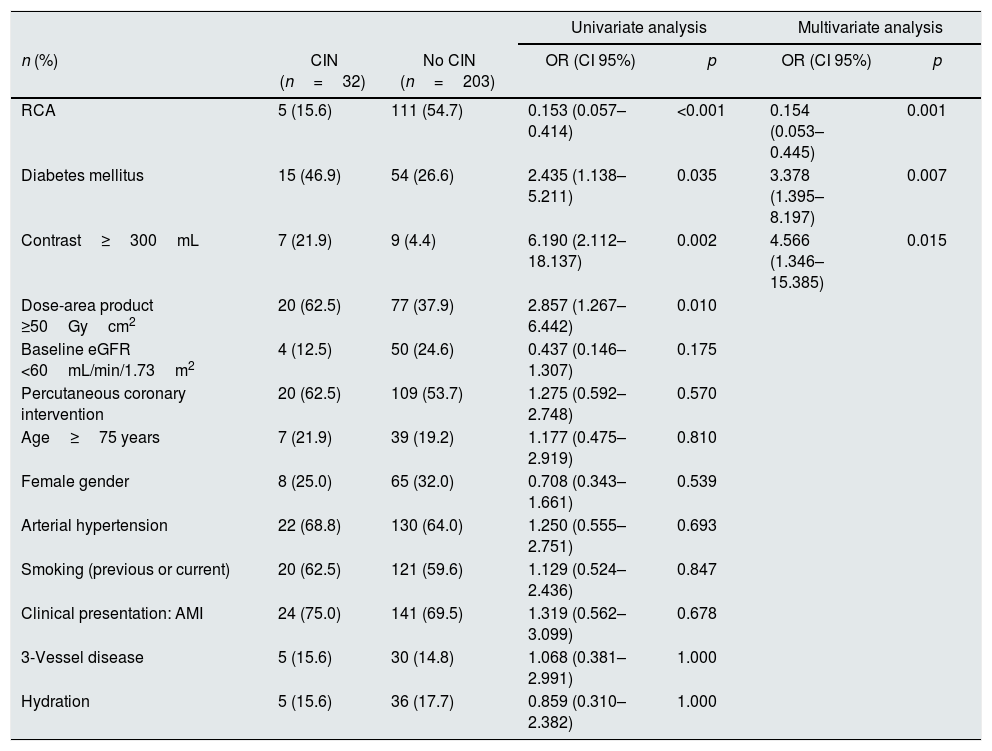
Results from logistic regression analysis to detect independent predictors of CIN (increase in serum creatinine ≥0.5mg/dL or ≥25%), ACR shows to be a protective factor (OR: 0.154; 95% CI: 0.053–0.445, p=0.001). Likewise, both diabetes mellitus (OR: 3.378, 95% CI: 1.395–8.197, p=0.007) and contrast volume administrated ≥300mL (OR: 4.566, 95% CI: 1.346–15.385; p=0.015) are risk factors for CIN (see Table 5).
Independent predictors of CIN (increase in serum creatinine ≥0.5mg/dL or ≥25%).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| n (%) | CIN (n=32) | No CIN (n=203) | OR (CI 95%) | p | OR (CI 95%) | p |
| RCA | 5 (15.6) | 111 (54.7) | 0.153 (0.057–0.414) | <0.001 | 0.154 (0.053–0.445) | 0.001 |
| Diabetes mellitus | 15 (46.9) | 54 (26.6) | 2.435 (1.138–5.211) | 0.035 | 3.378 (1.395–8.197) | 0.007 |
| Contrast≥300mL | 7 (21.9) | 9 (4.4) | 6.190 (2.112–18.137) | 0.002 | 4.566 (1.346–15.385) | 0.015 |
| Dose-area product ≥50Gycm2 | 20 (62.5) | 77 (37.9) | 2.857 (1.267–6.442) | 0.010 | ||
| Baseline eGFR <60mL/min/1.73m2 | 4 (12.5) | 50 (24.6) | 0.437 (0.146–1.307) | 0.175 | ||
| Percutaneous coronary intervention | 20 (62.5) | 109 (53.7) | 1.275 (0.592–2.748) | 0.570 | ||
| Age≥75 years | 7 (21.9) | 39 (19.2) | 1.177 (0.475–2.919) | 0.810 | ||
| Female gender | 8 (25.0) | 65 (32.0) | 0.708 (0.343–1.661) | 0.539 | ||
| Arterial hypertension | 22 (68.8) | 130 (64.0) | 1.250 (0.555–2.751) | 0.693 | ||
| Smoking (previous or current) | 20 (62.5) | 121 (59.6) | 1.129 (0.524–2.436) | 0.847 | ||
| Clinical presentation: AMI | 24 (75.0) | 141 (69.5) | 1.319 (0.562–3.099) | 0.678 | ||
| 3-Vessel disease | 5 (15.6) | 30 (14.8) | 1.068 (0.381–2.991) | 1.000 | ||
| Hydration | 5 (15.6) | 36 (17.7) | 0.859 (0.310–2.382) | 1.000 | ||
RCA: rotational coronary angiography; eGFR: estimated glomerular filtration; AMI: acute myocardial infarction; CI 95%: 95% confidence interval; CIN: contrast-induced nephropathy; OR: odds ratio.
Finally, the Poisson regression analysis reveals that RCA is associated with lower frequency of CIN defined as creatinine increase ≥0.5mg/dL or ≥25% (adjusted RR: 0.868, 95% CI: 0.794–0.949; p=0.002).
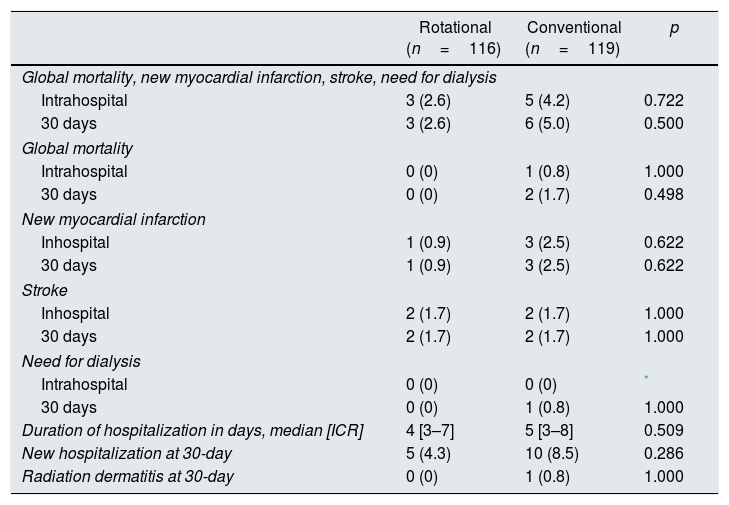
Clinical eventsClinical variables were not different in the two groups. Table 6 shows the data on clinical assessment variables.
Clinical events.
| Rotational (n=116) | Conventional (n=119) | p | |
|---|---|---|---|
| Global mortality, new myocardial infarction, stroke, need for dialysis | |||
| Intrahospital | 3 (2.6) | 5 (4.2) | 0.722 |
| 30 days | 3 (2.6) | 6 (5.0) | 0.500 |
| Global mortality | |||
| Intrahospital | 0 (0) | 1 (0.8) | 1.000 |
| 30 days | 0 (0) | 2 (1.7) | 0.498 |
| New myocardial infarction | |||
| Inhospital | 1 (0.9) | 3 (2.5) | 0.622 |
| 30 days | 1 (0.9) | 3 (2.5) | 0.622 |
| Stroke | |||
| Inhospital | 2 (1.7) | 2 (1.7) | 1.000 |
| 30 days | 2 (1.7) | 2 (1.7) | 1.000 |
| Need for dialysis | |||
| Intrahospital | 0 (0) | 0 (0) | * |
| 30 days | 0 (0) | 1 (0.8) | 1.000 |
| Duration of hospitalization in days, median [ICR] | 4 [3–7] | 5 [3–8] | 0.509 |
| New hospitalization at 30-day | 5 (4.3) | 10 (8.5) | 0.286 |
| Radiation dermatitis at 30-day | 0 (0) | 1 (0.8) | 1.000 |
ICR: interquartile range.
The most significant findings of our study were: (a) for the first time in the medical literature it was shown that in ACS patients treated with invasive coronary procedures, the RCA was associated with less incidence of CIN than CCA; (b) regression analysis shows that despite baseline differences between the two study groups, the use of ACR was associated with less development of CIN than CCA, (c) the presence of diabetes mellitus and the administration of at least 300amL of iodinated contrast during the performance of invasive coronary procedures were independent predictors of CIN.
Published data on the incidence of CIN are variable ranging from 1 to 33%.1–4 Such variability may be related to the different criteria used to define the CIN and also to the lack of strict protocols for detection of CIN.32 One of the strengths of the present study is the exhaustive evaluation performed aiming to detect CIN. All patients were evaluated for CIN, which gives us a realistic idea of the incidence of CIN in patients treated with CCA and in with RCA. This may be the reason why the incidence of CIN reported in our work is superior to that of some studies with less intense screening for CIN detection. In our study, 3 different criteria were used to identify CIN.21–23 The criterion used for CIN, as the primary endpoint, was an increase in creatinine ≥0.5mg/dL or ≥25% with respect to the baseline level between 48 and 72h after the procedure; this definition is the most associated with the occurrence of adverse events.21,33 Nevertheless, 2 additional criteria of CIN diagnosis were used as secondary endpoint variables, CIN with AKI-IC criteria and CIN with criterion of eGFR.22,23 Independently of the definitions used, the incidence of CIN was less frequent in patients undergoing RCA than CCA.
The present work shows that the use RCA allows a reduction in the amount of contrast used, both in diagnostic and combined procedures (diagnostic and therapeutic). The vast majority of the comparative studies of RCA and CCA were restricted only to the scenario of diagnostic coronary angiography, which is far from the usual clinical practice, in which usually a coronary angioplasty is performed immediately after the diagnostic procedure.34 Our data confirm that the performance of RCA followed or not by ad hoc angioplasty continues to show a reduction in the total volume of iodinated contrast used in any type of invasive coronary procedure. Analysis of independent predictors for the development of CIN shows that in addition to the presence of diabetes mellitus, the administration of high amounts of contrast (≥300mL) was a risk factor for CIN. This reaffirms the idea that the use of ACR allows to reduce CIN, by saving in the amount of contrast utilized. However, our results do not show that renal dysfunction was an independent predictor of CIN and this may be due to the limited number of patients with renal dysfunction included in the study.
The RCA is a simple and reproducible technique without great differences with respect to the conventional procedure. The catheters used and the technique of puncture and cannulation of the coronary arteries are similar in RCA and CCA; so, it would be easy to generalize its use in routine clinical practice.18,19,35,36 However, it should be taken into consideration that in RCA since the injection required to visualize each coronary artery requires a greater amount of contrast, it is necessary to be extremely careful to obtain a coaxial cannulation of the coronary to avoid inducing possible dissections of the coronary arteries. However, in line with the published evidence18,19,34 the CINERAMA Study has not observed an increase in complications related to the procedure.
The CIN is closely related to morbidity and mortality and therefore it has an impact on health cost becoming a serious health problem. The main contribution of our study is to show that a strategy to save contrast such as the use of RCA, is associated with reduction of CIN with all these negative consequences. The incidence of CIN is high, any improvement to reduce CIN is of great clinical relevance. Although future clinical trials are needed to confirm our results, the biological plausibility of our strategy as well as the simplicity of the technique could have a broad impact on clinical practice.
The RCA is a technique that could save money for the health system. It is foreseeable that the reduction in the incidence of CIN would result in lower healthcare costs, as shown in the study by Aubry et al.37 In this study, which evaluated more than one million hospitalizations in the French national health system, it was observed that patients who developed CIN had a much longer hospitalization (20.5 vs. 4.7 days; p<0.001) and with a much higher cost (15,654 vs. 3352 euros, p<0.001). Furthermore, it is expected that savings would not be limited only to hospital stay, but also resulting from the lower morbidity and mortality, so that the reduction of total costs of patients care could be even greater.
Study limitationsFirst, this study is an observational and prospective analysis (cohort study) with the biases inherent to this type of study. However, it is the first study to evaluate the impact of RCA on the development of the CIN. Second, the absence of randomization led to a non-homogeneous distribution of baseline variables (age, sex, eGFR, etc.) and therapies (percentage of implanted stents, etc.) between the two study groups; this could have influenced the incidence of the primary assessment variable (CIN). However, after adjusting for confounding factors, the results are favorable to the RCA. Third, our data refer to the population of the Canary Islands and, therefore, cannot be fully extrapolated to other geographical areas. Fourth, the procedure protocol of the CCA may not be identical in the different units of interventional cardiology. However, we consider that the CCA protocol may be considered conservative in terms of contrast administration, limitations in the performance of ventriculographies and the initial number of angiographic projections for each coronary artery and, for this reason, the final volume of contrast administered in the CCA group should not be overestimated. Fifth, the clinical follow-up of the patients was limited in time (one month), which may be related to the absence of differences in the clinical assessment variables between the study groups.
ConclusionsThe use of RCA in patients with ACS on invasive treated was associated with less administration of iodinated contrast, which resulted in a lower incidence of CIN. Further randomized clinical studies are required both in the ACS and in other clinical scenarios, to confirm the results presented in this work.
Conflict of interestsThe authors declare that they have no conflicts of interest.
Please cite this article as: Fernández-Rodríguez D, Grillo-Pérez JJ, Pérez-Hernández H, Rodríguez-Esteban M, Pimienta R, Acosta-Materán C, et al. Evaluación prospectiva del desarrollo de nefropatía inducida por contraste en pacientes con síndrome coronario agudo tratados con angiografía coronaria rotacional vs. angiografía coronaria convencional: Estudio CINERAMA. Nefrologia. 2018;38:169–178.