In routine clinical practice, the prescription of vitamin D analogues (VDA) in patients with chronic kidney disease (CKD) is often associated with a decline of the estimated renal function. The reason for this is not fully understood.
AimsTo analyze the effects of VDA discontinuation in advanced CKD and to determine the factors associated with changes in renal function.
Material and methodsRetrospective cohort study of adult patients with advanced CKD. The case subgroup was treated with VDA and this medication was discontinued at baseline (the first visit). The control subgroup was not treated with VDA and they were selected according to comparability principles for CKD progression by propensity score matching. The primary outcome measure was a change to both the estimated glomerular filtration rate (MDRD-GFR) and the measured glomerular filtration rate (mGFR by combined creatinine and urea clearances). Baseline parameters related to mineral metabolism and creatinine generation were analyzed as potential determinants of renal function changes.
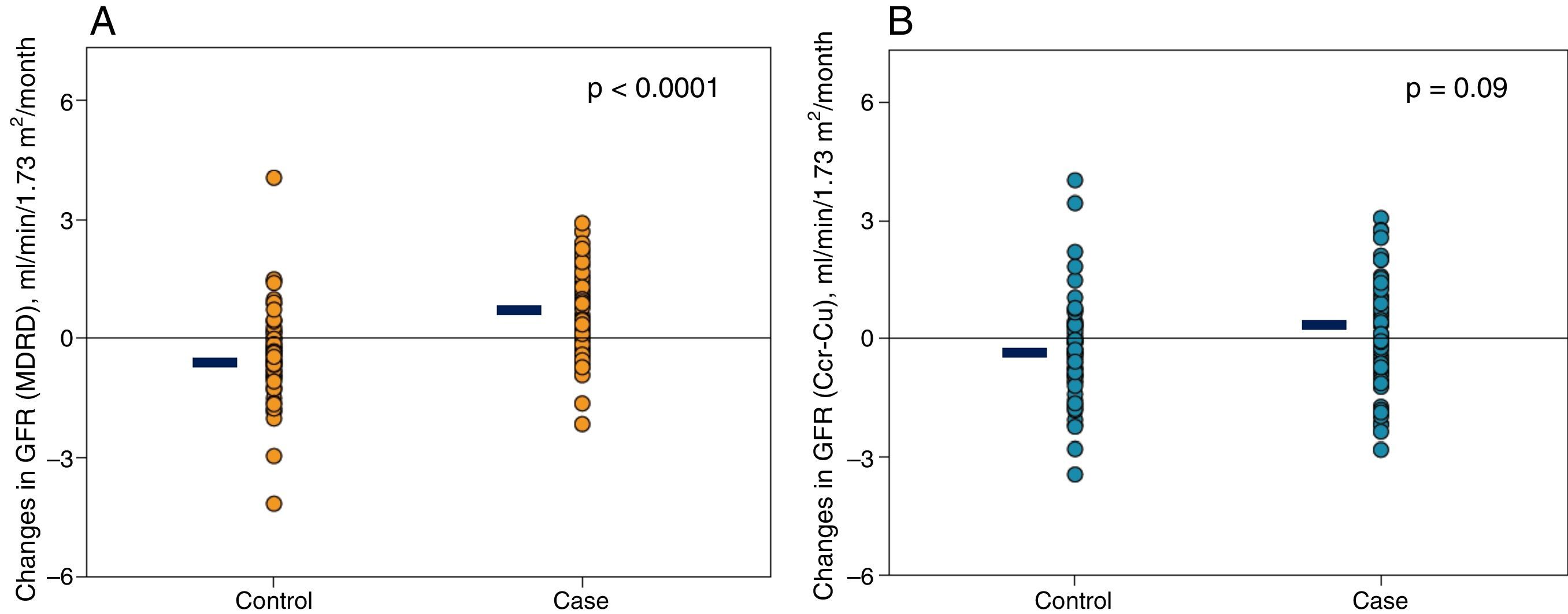
ResultsThe study sample consisted of 67 cases and 67 controls. Renal function improved in 67% of cases and worsened in 72% of controls (p<0.0001). Changes in MDRD-GFR for the case subgroup and the control subgroup were +0.455±0.997 vs. −0.436±1.103ml/min/1.73m2/month (p<0.0001), respectively. Total creatinine excretion was slightly higher in cases than in controls but the difference was not significant.
According to multivariate logistic and linear regression analyses, baseline total serum calcium was one of the best determinants of both renal function recovery (Odds ratio=3.49; p=0.001), and of the extent of renal function recovery (beta=0.276; p=0.001).
ConclusionsDiscontinuation of VDA treatment in CKD patients is associated with significant recovery of estimated renal function. The extent of these changes is mainly associated with baseline total serum calcium.
En la práctica clínica habitual la prescripción de análogos de vitamina D (AVD) en la enfermedad renal crónica (ERC) se asocia con frecuencia a un descenso de la función renal estimada cuyo origen no es bien conocido.
ObjetivosAnalizar el efecto de la suspensión de un tratamiento previo con AVD en ERC avanzada, y determinar los factores asociados con los cambios de función renal.
Material y métodosEstudio de cohorte retrospectivo en pacientes adultos incidentes con ERC avanzada. El subgrupo caso estaba siendo tratado con AVD y esta medicación fue suspendida en la primera visita. El subgrupo control no había sido tratado con AVD y fueron elegidos por criterios de coincidencia para datos relevantes relacionados con la progresión de la ERC. La variable de resultado principal fue el cambio de filtrado glomerular, tanto el estimado (FG-MDRD) como el medido (media del aclaramiento de creatinina y urea), con respecto al siguiente control analítico. Parámetros basales relacionados con el metabolismo mineral y la generación de creatinina fueron analizados como determinantes potenciales de los cambios de la función renal.
ResultadosSe incluyeron 67 pacientes casos y otros 67 controles. El 67% de los casos mejoró la función renal, mientras que el 72% de los controles empeoró (p<0,0001). El cambio FG-MDRD en casos y controles fue +0,455±0,997 vs. −0,436±1,103ml/min/1,73 m2/mes (p<0,0001), respectivamente. La excreción total de creatinina era ligeramente superior en los casos, pero la diferencia con respecto a los controles no fue significativa.
Por regresión logística y lineal multivariante, el calcio sérico total basal fue uno de los principales determinantes tanto de la recuperación de la función renal (odds ratio=3,49; p=0,001), como de la magnitud de esta recuperación (beta=0,276; p=0,001).
ConclusionesLa suspensión de AVD en pacientes con ERC se asocia con una mejoría significativa de la función renal estimada. La magnitud de estos cambios se relaciona principalmente con la calcemia basal.
Analogues or active forms of vitamin D (VDA) (calcitriol, paricalcitol, 22-oxacalcitriol, etc.) are an essential part of the treatment of bone-mineral disease associated with chronic kidney disease (CKD). In addition to its recognized therapeutic benefits on hyperparathyroidism, numerous experimental and clinical studies have observed that these drugs may also have pleiotropic effects including inhibition of the renin-angiotensin system1–4 and modulation of the inflammation and fibrogenesis porcesses,5–11 that in theory could confer renal and cardiovascular protective effects.
A clinically demonstrable effect of the prescription of VDA in patients with CKD is the significant reduction of proteinuria.1–4 However, it has not been demonstrated that these drugs improve some relevant cardiovascular parameters.12,13 In addition, the effect of VDA on survival in CKD is controversial and inadequately evaluated.14–18 Despite the reduction in proteinuria after VDA administration, it is not uncommon to observe a significant reduction in renal function.1,4,12,19–25
This adverse effect, usually reversible after drug discontinuation, has been interpreted by some researchers as an alteration in the creatinine metabolism (increase in endogenous generation), based on the results of studies that presented methodological irregularities and incomplete analysis of results.26–28
The objectives of the present study were: to analyze the changes in renal function after the discontinuation of VDA in patients with advanced CKD (ACKD), to determine the factors associated with the improvement of renal function (reversibility of the possible adverse effect of the drug), and to provide alternative explanations to those already published on the origin of this adverse effect and propose some practical measures to avoid undesirable effects of VDA in the ACKD.
Material and methodsCohort study with retrospective data collection, in adult patients, without racial differences (all Caucasians), incidents in the ACKD outpatient clinic during the period between January 2013 and December 2015.
The subgroup of cases included adult patients who had been referred to the ACKD clinic from other outpatient departments of Nephrology or Internal Medicine for progressive deterioration of renal function reaching stages 4 or 5 (GFR<30ml/min/1.73m2 in all patients), and who were on VDA (calcitriol or paricalcitol) for at least 3 months before referral. The VDA was discontinued at the first visit to the ACKD following a uniform protocol. This modification of the treatment has been our practice for more than 10 years for strictly clinical reasons based on the repeated observation of improvement in renal function, and patients are informed of this change in the treatment. Phosphate binders or sodium bicarbonate were also prescribed according to serum levels. Patients who had experienced a recent exacerbation of CKD were not included.
In addition to the relevant demographic and clinical data, the following biochemical parameters were measured by conventional clinical laboratory methods (Advia Chemistry, Siemens Healthcare Diagnostics, New York, USA): total calcium, phosphorus, magnesium (colorimetry with blue xylidil), alkaline phosphatase, albumin, PTH (molecule 7–84, automated chemiluminescent immunoassay DiaSorin, Italy), bicarbonate and ionized calcium (ABL800 FLEX, Radiometer Ibérica, Spain). In urine collected for 24h, the following parameters were determined: proteinuria (expressed as g/g of creatinine), calcium, phosphorus and total excretion of creatinine and urea.
The glomerular filtration rate was estimated using the abbreviated formula MDRD-429 and, in addition, it was measured by urea and creatinine clearance (half of the sum of both clearances).30 All the measurements were performed in the same laboratory (Clinical Analysis Service of Infanta Cristina Hospital) and the creatinine calibrations and traceability were carried out in accordance with the recommendations of international standards (LWG-NKDEP). The rate of protein catabolism was calculated through the urinary excretion of urea nitrogen using the formula of Maroni et al.31
The biochemical analysis was repeated on the second visit, 2–3 months after the first visit.
The subgroup of controls were adult patients from the same cohort who had been referred to the ACKD clinic, but who were not on treatment with VDA. The selection procedure was based on matching criteria for relevant baseline data related to the progression of CKD (see below in statistical methods). According to previous studies in our ACKD population, the factors most significantly related to the rate of progression of renal failure are: age, gender, body mass index, systolic blood pressure, proteinuria and treatment with drugs with a potential iatrogenic effect (in addition to non-steroidal anti-inflammatories, allopurinol in certain patients, fibrates, double blockade of the renin-angiotensin system and biphosphonates).
We did not include any patient (neither in cases nor in controls) who was being treated or who had been suspended at the first visit any of these drugs.
All control patients were also prescribed phosphorus binders or sodium bicarbonate according to requirements, and they underwent the same biochemical study as in the cases including a second visit 2–3 months later.
In both, cases and controls, the total theoretical urinary creatinine excretion was estimated basally and in the following visit according to the formulas described by Ix et al.32 that take into account age, gender and race. To normalize the total actual excretion of creatinine in each patient, this was expressed as a percentage of the estimated theoretical excretion.
Study design and statistical analysisThis study analyzes the effect of the discontinuation of VDA on the renal function in a cohort of patients exposed to the same risk factors for CKD progression as controls.
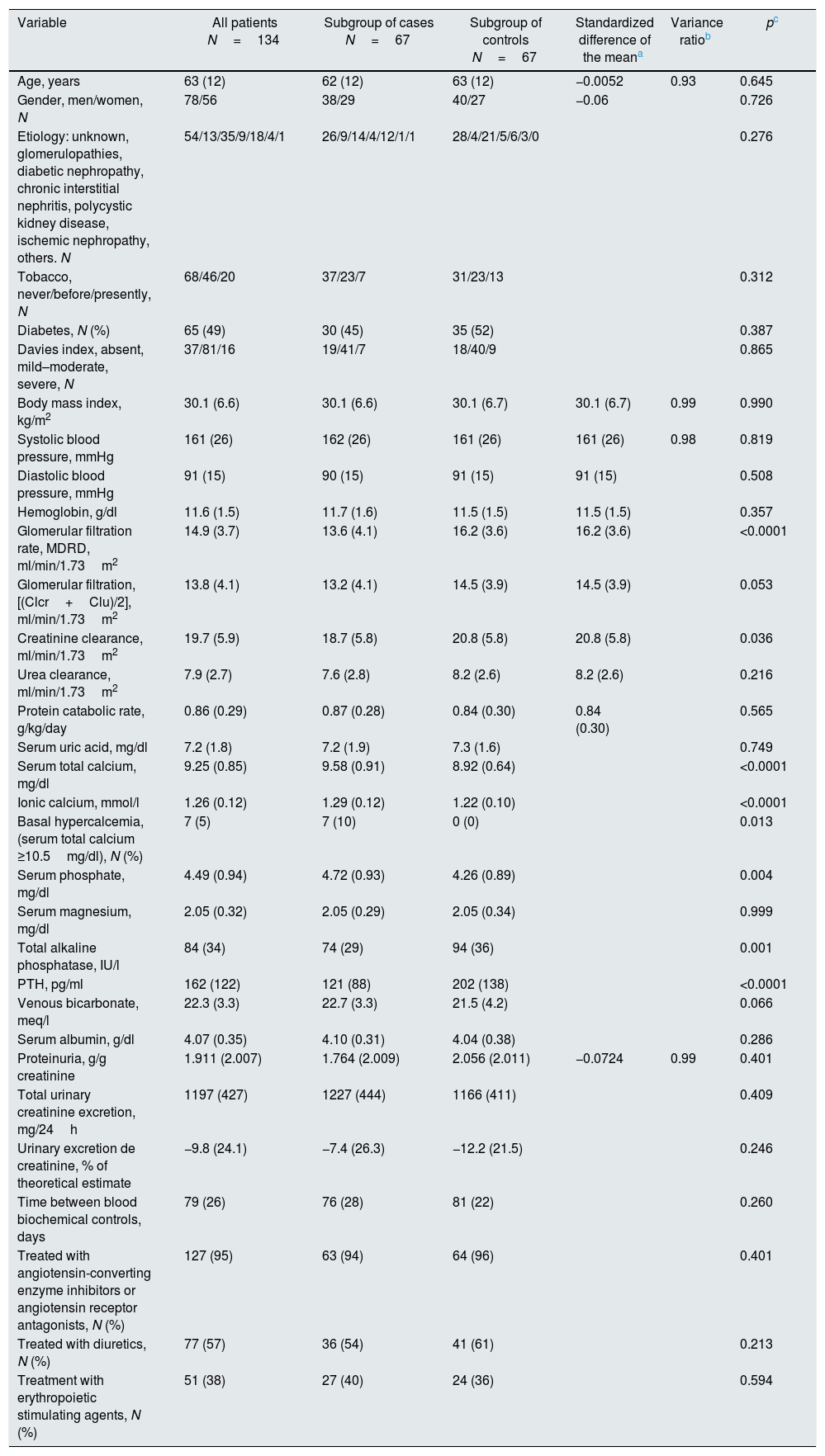
The selection procedure of the controls was carried out by pairing by propensity score, having previously excluded those patients in whom other drugs with potential influence on the deterioration of renal function were modified. The propensity score was obtained by logistic regression in which they were included as “covariables” for predicting the progression of renal failure: age, sex, body mass index, systolic blood pressure and proteinuria. The standardized differences of the means of these variables and the quotient of variances in the continuous “covariables” are shown in Table 1.
Characteristics of patients according to subgroups. The variables that were chosen for the selection of controls show the standardized differences of the mean and the quotient of variances with respect to the values of cases.
| Variable | All patients N=134 | Subgroup of cases N=67 | Subgroup of controls N=67 | Standardized difference of the meana | Variance ratiob | pc |
|---|---|---|---|---|---|---|
| Age, years | 63 (12) | 62 (12) | 63 (12) | −0.0052 | 0.93 | 0.645 |
| Gender, men/women, N | 78/56 | 38/29 | 40/27 | −0.06 | 0.726 | |
| Etiology: unknown, glomerulopathies, diabetic nephropathy, chronic interstitial nephritis, polycystic kidney disease, ischemic nephropathy, others. N | 54/13/35/9/18/4/1 | 26/9/14/4/12/1/1 | 28/4/21/5/6/3/0 | 0.276 | ||
| Tobacco, never/before/presently, N | 68/46/20 | 37/23/7 | 31/23/13 | 0.312 | ||
| Diabetes, N (%) | 65 (49) | 30 (45) | 35 (52) | 0.387 | ||
| Davies index, absent, mild–moderate, severe, N | 37/81/16 | 19/41/7 | 18/40/9 | 0.865 | ||
| Body mass index, kg/m2 | 30.1 (6.6) | 30.1 (6.6) | 30.1 (6.7) | 30.1 (6.7) | 0.99 | 0.990 |
| Systolic blood pressure, mmHg | 161 (26) | 162 (26) | 161 (26) | 161 (26) | 0.98 | 0.819 |
| Diastolic blood pressure, mmHg | 91 (15) | 90 (15) | 91 (15) | 91 (15) | 0.508 | |
| Hemoglobin, g/dl | 11.6 (1.5) | 11.7 (1.6) | 11.5 (1.5) | 11.5 (1.5) | 0.357 | |
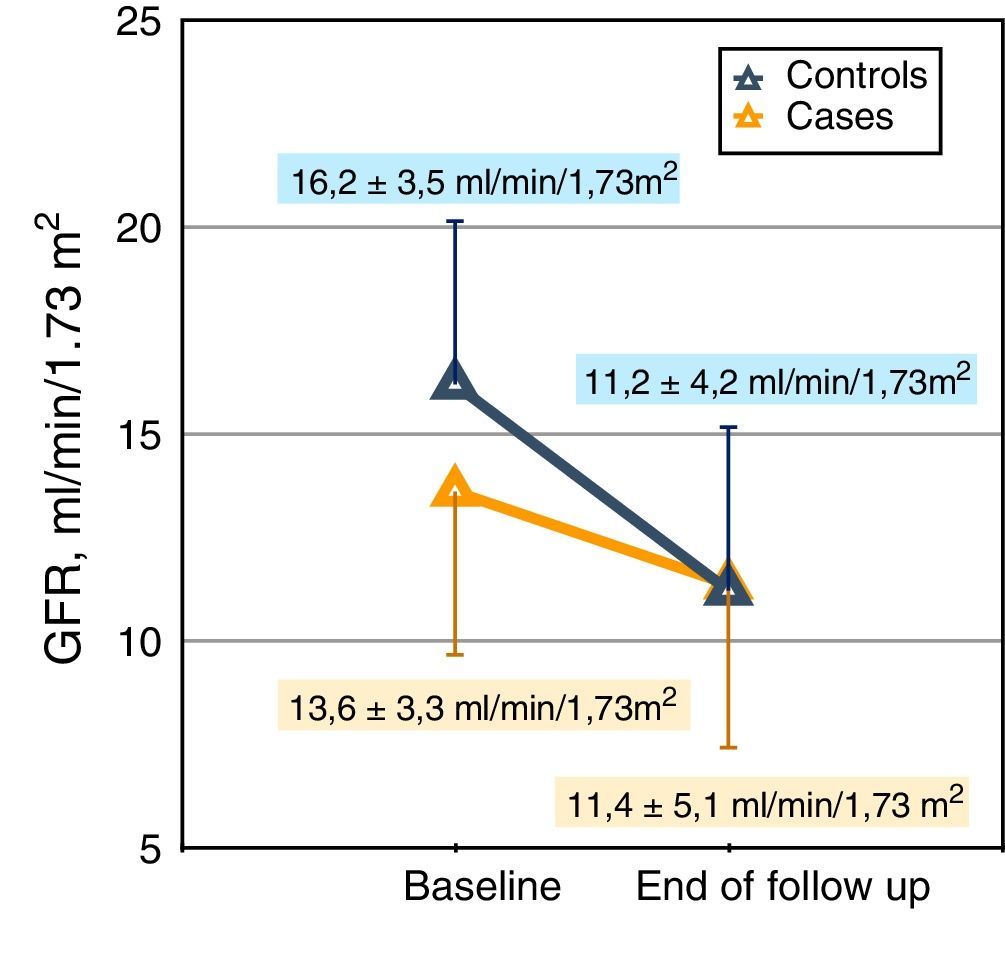
| Glomerular filtration rate, MDRD, ml/min/1.73m2 | 14.9 (3.7) | 13.6 (4.1) | 16.2 (3.6) | 16.2 (3.6) | <0.0001 | |
| Glomerular filtration, [(Clcr+Clu)/2], ml/min/1.73m2 | 13.8 (4.1) | 13.2 (4.1) | 14.5 (3.9) | 14.5 (3.9) | 0.053 | |
| Creatinine clearance, ml/min/1.73m2 | 19.7 (5.9) | 18.7 (5.8) | 20.8 (5.8) | 20.8 (5.8) | 0.036 | |
| Urea clearance, ml/min/1.73m2 | 7.9 (2.7) | 7.6 (2.8) | 8.2 (2.6) | 8.2 (2.6) | 0.216 | |
| Protein catabolic rate, g/kg/day | 0.86 (0.29) | 0.87 (0.28) | 0.84 (0.30) | 0.84 (0.30) | 0.565 | |
| Serum uric acid, mg/dl | 7.2 (1.8) | 7.2 (1.9) | 7.3 (1.6) | 0.749 | ||
| Serum total calcium, mg/dl | 9.25 (0.85) | 9.58 (0.91) | 8.92 (0.64) | <0.0001 | ||
| Ionic calcium, mmol/l | 1.26 (0.12) | 1.29 (0.12) | 1.22 (0.10) | <0.0001 | ||
| Basal hypercalcemia, (serum total calcium ≥10.5mg/dl), N (%) | 7 (5) | 7 (10) | 0 (0) | 0.013 | ||
| Serum phosphate, mg/dl | 4.49 (0.94) | 4.72 (0.93) | 4.26 (0.89) | 0.004 | ||
| Serum magnesium, mg/dl | 2.05 (0.32) | 2.05 (0.29) | 2.05 (0.34) | 0.999 | ||
| Total alkaline phosphatase, IU/l | 84 (34) | 74 (29) | 94 (36) | 0.001 | ||
| PTH, pg/ml | 162 (122) | 121 (88) | 202 (138) | <0.0001 | ||
| Venous bicarbonate, meq/l | 22.3 (3.3) | 22.7 (3.3) | 21.5 (4.2) | 0.066 | ||
| Serum albumin, g/dl | 4.07 (0.35) | 4.10 (0.31) | 4.04 (0.38) | 0.286 | ||
| Proteinuria, g/g creatinine | 1.911 (2.007) | 1.764 (2.009) | 2.056 (2.011) | −0.0724 | 0.99 | 0.401 |
| Total urinary creatinine excretion, mg/24h | 1197 (427) | 1227 (444) | 1166 (411) | 0.409 | ||
| Urinary excretion de creatinine, % of theoretical estimate | −9.8 (24.1) | −7.4 (26.3) | −12.2 (21.5) | 0.246 | ||
| Time between blood biochemical controls, days | 79 (26) | 76 (28) | 81 (22) | 0.260 | ||
| Treated with angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists, N (%) | 127 (95) | 63 (94) | 64 (96) | 0.401 | ||
| Treated with diuretics, N (%) | 77 (57) | 36 (54) | 41 (61) | 0.213 | ||
| Treatment with erythropoietic stimulating agents, N (%) | 51 (38) | 27 (40) | 24 (36) | 0.594 |
In the continuous variables (±SD).
Once punctuated, the choice of pairs in a 1:1 ratio was made by a corresponding element (nearest neighbor) without replacement and weighing of the choice in relation to distance (width of calibration less than 0.2). After pairing, both groups showed a very similar distribution of propensity scores.
The following renal parameters were compared between cases and controls: 1. Change of evolutionary trend of renal function (worsening or recovery of renal function) in the subsequent control. 2. Magnitude of changes in renal function (estimated GFR and measured GFR, expressed as ml/min/month), proteinuria, mineral metabolism parameters and total urinary creatinine excretion (percentage of the theoretical estimate) in the next analytical control. 3. Slope of the linear regression between glomerular filtration and time of evolution, until the start of dialysis or end of follow-up, expressed in ml/min/month. 4. Curves of survival without dialysis in cases and controls.
Parametric or nonparametric tests were used descriptive comparison of the continuous variables depending on their characteristics. The χ2 test was used to compare categorical variables. The Kolmogorov–Smirnov was used to determine if the distribution of a quantitative variable followed a normal pattern.
In the total study cohort, the best determinants of favorable change (recovery of renal function in the following analytical control) were analyzed by multivariate logistic regression. The best determinants of the magnitude of these changes in renal function were also investigated by multiple linear regression.
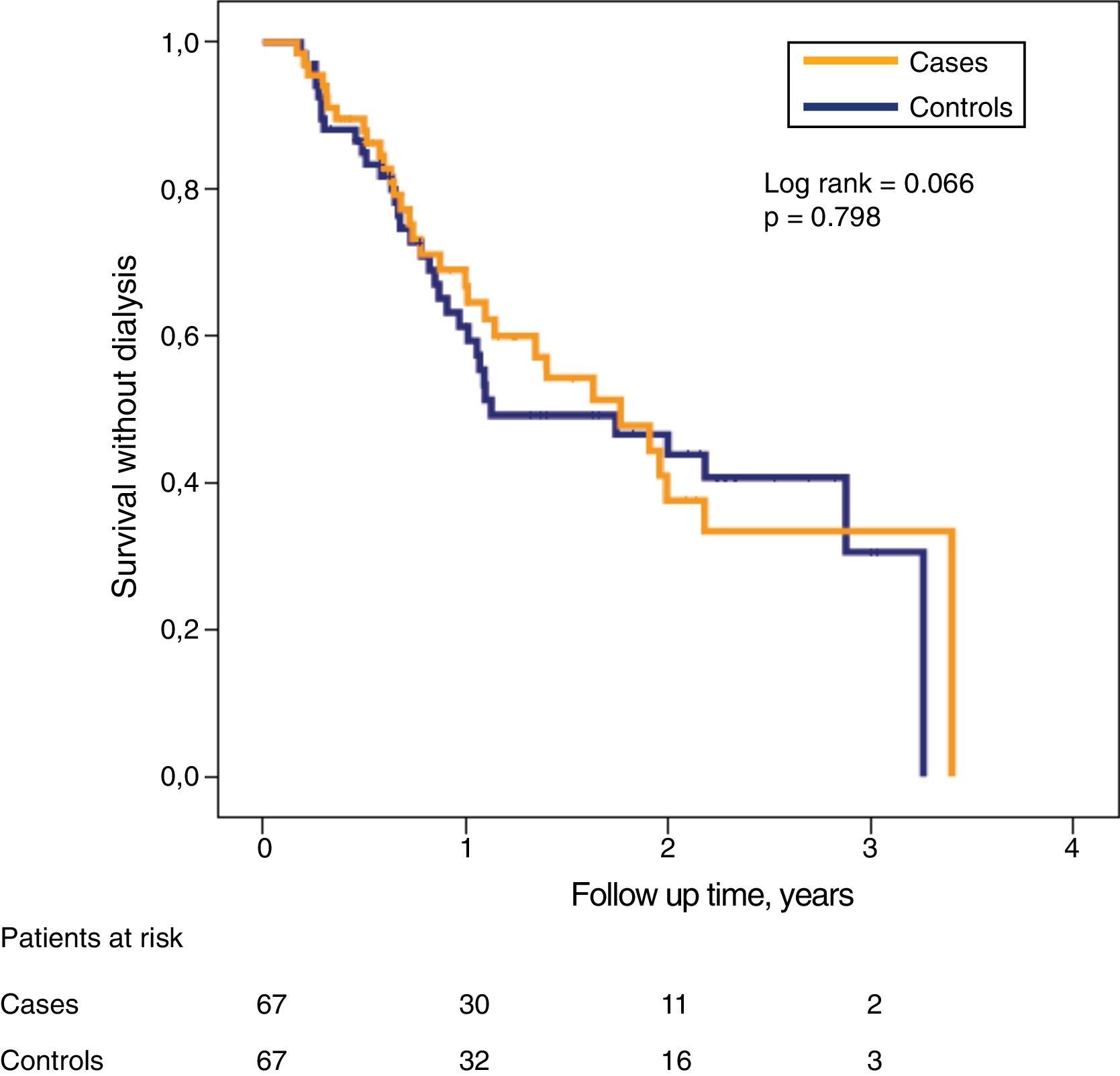
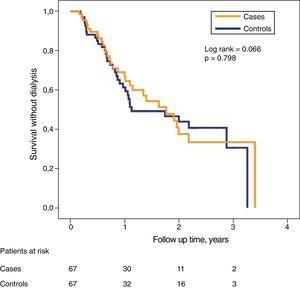
Differences in dialysis free survival between case patients and controls were analyzed with Kaplan–Meier curves and compared with the Mantel–Haenszel logarithmic rank test. The influence of the main study variables on the combined development of dialysis onset or mortality (survival without dialysis) was to analyze with a multivariate model of proportional risk of Cox, which was adjusted by the process of conditional progressive elimination backwards. The patients were censored at the time of death, loss of follow-up, initiation of dialysis or end of follow-up.
A p<0.05 was considered statistically significant, and all are two tails p values. The statistical analyses were performed with IBM SPSS Statistics 21.0 software (IBM Corp., Armonk, USA).
ResultsBaseline characteristics of patientsA total of 266 patients were referred and studied for the first time in the ACKD clinic during the inclusion period consultation. Of these, 134 patients were included in the study, 67 “case” patients and 67 other “control” patients. The demographic, clinical and biochemical characteristics are shown in Table 1.
The case patients were treated with the following VDA: 60 patients with paricalcitol (dose of 1μg daily in 56 patients and dose of 2μg daily in 4 patients), and 7 patients with calcitriol (4 with dose of 0.25μg daily and 3 with 0.50μg daily).
The most notable differences between these 2 subgroups were: the significantly reduced renal function of the cases, possibly related to the accelerated and unforeseen deterioration of glomerular filtration in those who were being treated with VDA, and the expected biochemical differences related to the use of VDA such as higher calcemia and phosphatemia, and lower concentrations of alkaline phosphatase and PTH in cases as compared to controls.
There were no other significant differences in factors and parameters that could predict progression of CKD between both subgroups. The case patients had a slightly lower proteinuria than the controls and both the total urinary creatinine excretion and the percentage of excretion over the theoretical estimate were slightly higher in cases as compared to the controls, but these differences did not reach statistical significance.
The mean time elapsed between the 2 biochemical measurements was very similar between the two subgroups, and no differences were observed in the percentage of patients treated with other drugs of interest (diuretics, angiotensin converting enzyme inhibitors or angiotensin antagonists and erythropoietic stimulation agents).
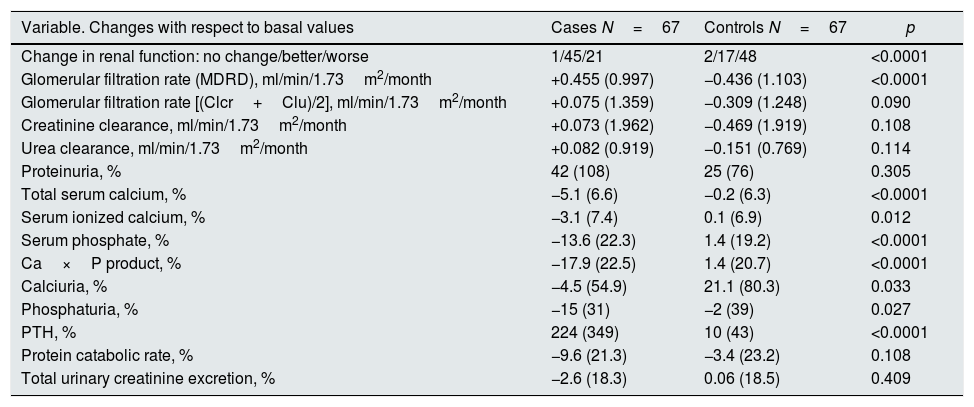
Differences in the progression of renal function and biochemical data between the two subgroupsTable 2 shows the changes observed in parameters evaluated in both subgroups.
Changes in biochemical parameters of interest in the first control according to groups.
| Variable. Changes with respect to basal values | Cases N=67 | Controls N=67 | p |
|---|---|---|---|
| Change in renal function: no change/better/worse | 1/45/21 | 2/17/48 | <0.0001 |
| Glomerular filtration rate (MDRD), ml/min/1.73m2/month | +0.455 (0.997) | −0.436 (1.103) | <0.0001 |
| Glomerular filtration rate [(Clcr+Clu)/2], ml/min/1.73m2/month | +0.075 (1.359) | −0.309 (1.248) | 0.090 |
| Creatinine clearance, ml/min/1.73m2/month | +0.073 (1.962) | −0.469 (1.919) | 0.108 |
| Urea clearance, ml/min/1.73m2/month | +0.082 (0.919) | −0.151 (0.769) | 0.114 |
| Proteinuria, % | 42 (108) | 25 (76) | 0.305 |
| Total serum calcium, % | −5.1 (6.6) | −0.2 (6.3) | <0.0001 |
| Serum ionized calcium, % | −3.1 (7.4) | 0.1 (6.9) | 0.012 |
| Serum phosphate, % | −13.6 (22.3) | 1.4 (19.2) | <0.0001 |
| Ca×P product, % | −17.9 (22.5) | 1.4 (20.7) | <0.0001 |
| Calciuria, % | −4.5 (54.9) | 21.1 (80.3) | 0.033 |
| Phosphaturia, % | −15 (31) | −2 (39) | 0.027 |
| PTH, % | 224 (349) | 10 (43) | <0.0001 |
| Protein catabolic rate, % | −9.6 (21.3) | −3.4 (23.2) | 0.108 |
| Total urinary creatinine excretion, % | −2.6 (18.3) | 0.06 (18.5) | 0.409 |
PTH: parathyroid hormone.
In continuous variables (±standard deviation).
A 67% of the case patients improved renal function in the second visit with respect to the baseline, while in 72% of the controls the renal function worsened.
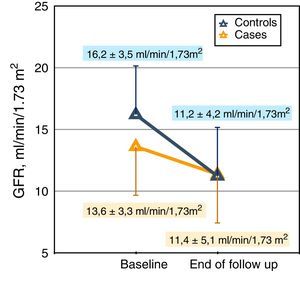
The changes in renal function estimated by the MDRD formula were very significant (Table 2 and Fig. 1A); if the glomerular filtration was measured by creatinine clearance, the differences were also appreciable, although they did not reach statistical significance (Table 2 and Fig. 1B).
Representation of individual changes in renal function. (A) Glomerular filtration rate estimated by MDRD in cases and controls patients. (B) Glomerular filtration rate (calculated as half of the sum of creatinine and urea clearance) in case and control patients. The horizontal black lines represent the mean of the respective values.
Other changes of interest were: the increase in proteinuria in both subgroups that was greater in cases, but without reaching significant differences with respect to controls; also relevant and significant were the reductions in total and ionized calcium, phosphatemia, calcium-phosphorus product, calciuria and phosphaturia in the case subgroup.
No significant differences were observed in the rate of protein catabolism, nor in the urinary excretion of creatinine between both subgroups.
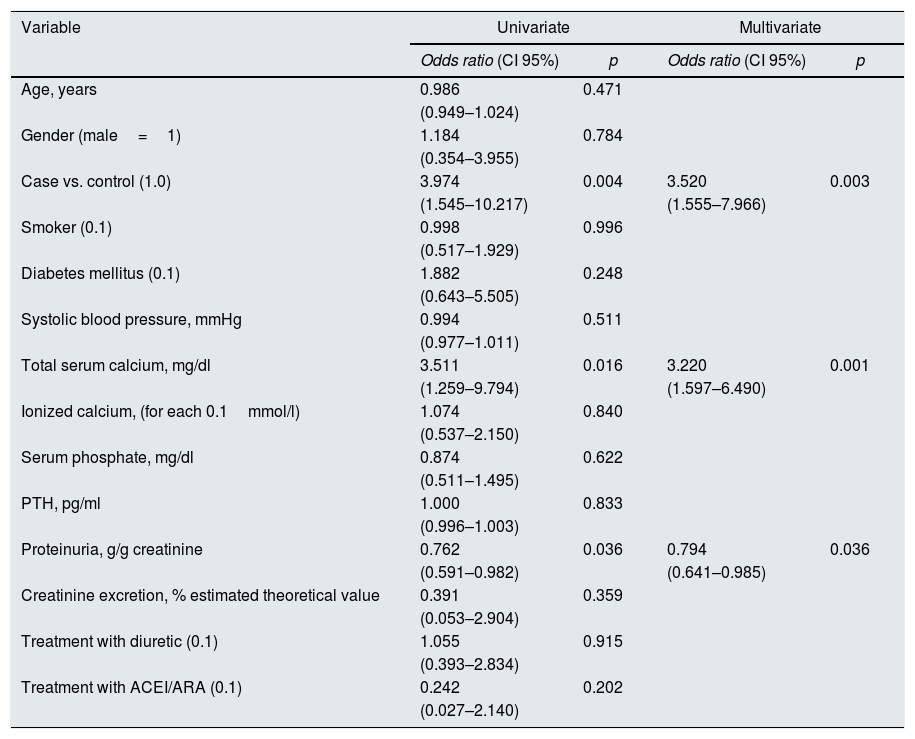
Determinants of the evolutionary trend and the magnitude of changes in renal function in the total cohort of patientsTable 3 shows the best determinants of a favorable trend of renal function according to univariate and multivariate logistic regression in the total study cohort.
Univariate and multivariate logistic regression. Determinants of the improvement of renal function in the second control (categorical value=1) versus worsening renal function (categorical value=0).
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| Odds ratio (CI 95%) | p | Odds ratio (CI 95%) | p | |
| Age, years | 0.986 | 0.471 | ||
| (0.949–1.024) | ||||
| Gender (male=1) | 1.184 | 0.784 | ||
| (0.354–3.955) | ||||
| Case vs. control (1.0) | 3.974 | 0.004 | 3.520 | 0.003 |
| (1.545–10.217) | (1.555–7.966) | |||
| Smoker (0.1) | 0.998 | 0.996 | ||
| (0.517–1.929) | ||||
| Diabetes mellitus (0.1) | 1.882 | 0.248 | ||
| (0.643–5.505) | ||||
| Systolic blood pressure, mmHg | 0.994 | 0.511 | ||
| (0.977–1.011) | ||||
| Total serum calcium, mg/dl | 3.511 | 0.016 | 3.220 | 0.001 |
| (1.259–9.794) | (1.597–6.490) | |||
| Ionized calcium, (for each 0.1mmol/l) | 1.074 | 0.840 | ||
| (0.537–2.150) | ||||
| Serum phosphate, mg/dl | 0.874 | 0.622 | ||
| (0.511–1.495) | ||||
| PTH, pg/ml | 1.000 | 0.833 | ||
| (0.996–1.003) | ||||
| Proteinuria, g/g creatinine | 0.762 | 0.036 | 0.794 | 0.036 |
| (0.591–0.982) | (0.641–0.985) | |||
| Creatinine excretion, % estimated theoretical value | 0.391 | 0.359 | ||
| (0.053–2.904) | ||||
| Treatment with diuretic (0.1) | 1.055 | 0.915 | ||
| (0.393–2.834) | ||||
| Treatment with ACEI/ARA (0.1) | 0.242 | 0.202 | ||
| (0.027–2.140) | ||||
ARA: angiotensin receptor antagonists; CI: 95%: 95% confidence interval; ACEI: angiotensin-converting enzyme inhibitors.
As compared with controls, case patients were 3½ times more likely to immediately improve kidney function than controls. In addition to proteinuria, the total serum calcium concentration determined the likelihood of immediate improvement of renal function.
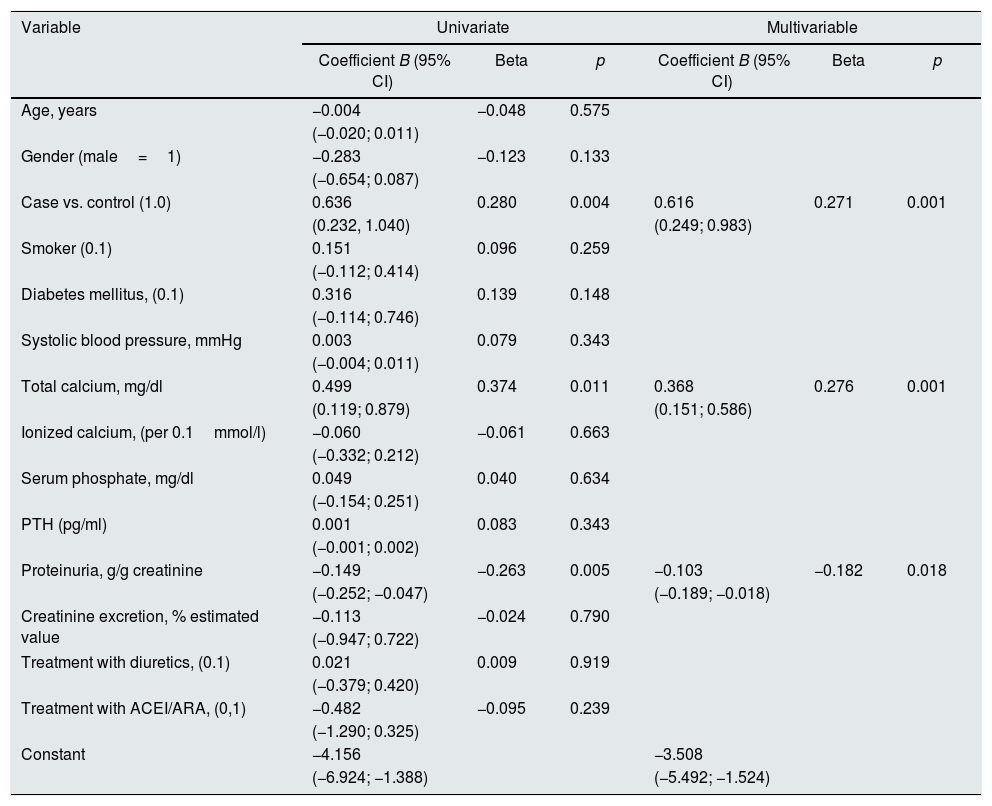
Table 4 shows the main determinants of the magnitude of change in glomerular filtration rate (MDRD). In addition to the suspension of VDA and proteinuria, basal total calcemia was also a significant determinant of these changes. That is, the higher the total basal calcemia, the greater the recovery of renal function after the suspension of VDA.
Multivariate linear regression. Determinants of the magnitude of changes in renal function (glomerular filtration rate MDRD in ml/min/1.73m2/month) in the second control.
| Variable | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
| Coefficient B (95% CI) | Beta | p | Coefficient B (95% CI) | Beta | p | |
| Age, years | −0.004 | −0.048 | 0.575 | |||
| (−0.020; 0.011) | ||||||
| Gender (male=1) | −0.283 | −0.123 | 0.133 | |||
| (−0.654; 0.087) | ||||||
| Case vs. control (1.0) | 0.636 | 0.280 | 0.004 | 0.616 | 0.271 | 0.001 |
| (0.232, 1.040) | (0.249; 0.983) | |||||
| Smoker (0.1) | 0.151 | 0.096 | 0.259 | |||
| (−0.112; 0.414) | ||||||
| Diabetes mellitus, (0.1) | 0.316 | 0.139 | 0.148 | |||
| (−0.114; 0.746) | ||||||
| Systolic blood pressure, mmHg | 0.003 | 0.079 | 0.343 | |||
| (−0.004; 0.011) | ||||||
| Total calcium, mg/dl | 0.499 | 0.374 | 0.011 | 0.368 | 0.276 | 0.001 |
| (0.119; 0.879) | (0.151; 0.586) | |||||
| Ionized calcium, (per 0.1mmol/l) | −0.060 | −0.061 | 0.663 | |||
| (−0.332; 0.212) | ||||||
| Serum phosphate, mg/dl | 0.049 | 0.040 | 0.634 | |||
| (−0.154; 0.251) | ||||||
| PTH (pg/ml) | 0.001 | 0.083 | 0.343 | |||
| (−0.001; 0.002) | ||||||
| Proteinuria, g/g creatinine | −0.149 | −0.263 | 0.005 | −0.103 | −0.182 | 0.018 |
| (−0.252; −0.047) | (−0.189; −0.018) | |||||
| Creatinine excretion, % estimated value | −0.113 | −0.024 | 0.790 | |||
| (−0.947; 0.722) | ||||||
| Treatment with diuretics, (0.1) | 0.021 | 0.009 | 0.919 | |||
| (−0.379; 0.420) | ||||||
| Treatment with ACEI/ARA, (0,1) | −0.482 | −0.095 | 0.239 | |||
| (−1.290; 0.325) | ||||||
| Constant | −4.156 | −3.508 | ||||
| (−6.924; −1.388) | (−5.492; −1.524) | |||||
ARA: angiotensin receptor antagonists; ACEI: angiotensin-converting enzyme inhibitors.
Negative or positive value means impairment or improvement of renal function, respectively.
Basal total serum calcium level was even greater determinant of improvement in renal function measured by creatine-urea clearances, (beta=0.466; p<.0.0001).
Long-term changes in both study subgroupsPatients from both subgroups were followed until: death, onset of dialysis, loss of follow-up or end of the study, during a median of 325 days (interquartile ranges 210–639 days). There were no significant differences in the follow-up time between cases (median 318 days) and controls (median 331 days).
The rate of decrease in glomerular filtration rate (slope of the linear regression between glomerular filtration rate calculated by MDRD formula and time) in the case and control subgroups was (median and interquartile ranges): −0.19 (−0.47; −0.006) and −0.39 (−0.76; −0.21)ml/min/month, respectively (p=0.001; Mann–Whitney test) (Fig. 2).
The comparison of the reduction in glomerular filtration rate measured by combined clearances in cases and controls did not reach statistical difference, (median and interquartile ranges): −0.17 (−0.37; −0.01) and −0.30 (−0.56; −0.07)ml/min/month, respectively (p=0.059, Mann–Whitney test).
During this period of follow-up, 31 patients from case subgroup needed to start dialysis, one patient died and there were no follow-up losses. In the control subgroup, 29 needed to start dialysis, 4 died and in 2 the follow-up was lost.
Despite the significant differences in glomerular filtration between both subgroups in the initial phase of the study, the probability of survival without need of dialysis was very similar (Fig. 3).
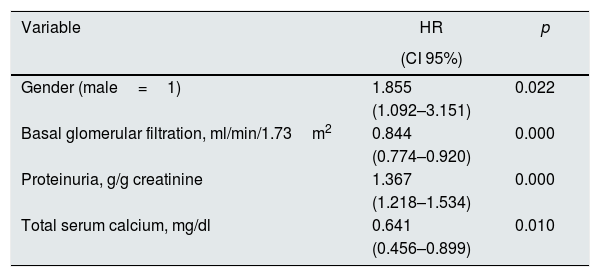
The Cox proportional risk regression model identified the covariates associated with worse combined outcome (death or onset of dialysis); these are shown in Table 5. In addition to the most expected variables (gender, baseline renal function, proteinuria), the total serum calcium, again, showed a significant association with survival without dialysis.
Cox proportional risk regression of association with death or onset of dialysis (survival without dialysis).
| Variable | HR | p |
|---|---|---|
| (CI 95%) | ||
| Gender (male=1) | 1.855 | 0.022 |
| (1.092–3.151) | ||
| Basal glomerular filtration, ml/min/1.73m2 | 0.844 | 0.000 |
| (0.774–0.920) | ||
| Proteinuria, g/g creatinine | 1.367 | 0.000 |
| (1.218–1.534) | ||
| Total serum calcium, mg/dl | 0.641 | 0.010 |
| (0.456–0.899) |
HR: hazard ratio or instantaneous risk ratio; 95% CI: 95% confidence intervals.
Variables included in the combined analysis but not selected for the best predictive equation: age, case or control group, smoker, diabetes mellitus, systolic blood pressure, serum phosphorus, ionic calcium, PTH, total urinary creatinine excretion, diuretic treatment, treatment with IECA/ARA.
The results of this study show that the discontinuation of a treatment with VDA in ACKD promptly improves the estimated renal function and slows down the rate of renal function deterioration. This change in the trend of CKD can be predicted by baseline calcemia: the higher the basal serum calcium, the more likely to recover renal function.
After the suspension of VDA, no significant changes were observed in total urinary creatinine excretion and there was no difference between cases and controls.
Since the first clinical trials on the use of VDA in CKD patients, it was observed that this treatment was associated with a significant decrease in renal function whose origin was unclear,20,21 and even this observation could also be demonstrated in experimental animals in a dose-dependent manner.22 However, in some other studies this reduction in glomerular filtration rate was not observed and the authors considered that the preservation of renal function was due to moderation of the prescribed doses of VDA and to the meticulous follow-up of their patients.33,34
In a more recent attempt to clarify this concerning problem, Agarwal et al.28 conducted a study in only 16 patients with CKD, the majority in stage 3, which consisted of administering 2g of paricalcitol daily for 7 days. However, the methodological problems of this study (number of patients, follow-up), analysis of the results (in some patients did the glomerular filtration rate measured by iodotalamate decrease significantly and in others did not) and interpretation (increase in creatinine production due to increased muscle mass) do not allow to clarify the issue of whether VDA affect renal function, although this study is the main reference in the literature.
In recent years we have observed an increase in early prescription (CKD stage 3–4) of VDA in our patients, in many cases pursuing theoretical pleiotropic benefits. The observation of unexpected deterioration of the renal function related to the administration of VDA and the subsequent referral to the ACKD clinic led us to a protocolized discontinuation of all VDA prescriptions of patients incidents in the ACKD out patient clinic, and evaluate the impact on the renal function.
In this study, the immediate improvement of renal function and the slower progression of renal function deterioration after the suspension of VDA compared with controls could not be explained by the changes in creatinine excretion. This hypothesis is proposed by some authors to attribute the increase in serum creatinine to an increase in muscle creatinine generation.26–28 The origin of this increase in creatinine is not very clear and should be taken into account for its correct interpretation that the urinary excretion of creatinine as an indicator of lean mass is not reliable in situations in which the glomerular filtration rate fluctuates (decrease and recovery) significantly in a short time, and the excretion of solutes is not in a steady state, as could have happened in the study by Agarwal et al.28 It is also not very likely that VDA may have a muscular anabolic effect so fast and effective as to significantly increase muscle mass and creatinine generation in a single week.
Another possible explanation could be related to an inhibitory effect of VDA on the tubular secretion of creatinine,27 similar to that caused by other drugs, such as cimetidine or trimethoprim, which elevate serum creatinine levels, thus causing underestimation of glomerular filtration rate. This mechanism could help to explain the differences that were observed in our study between the estimated glomerular filtration rate and the one measured by combined creatinine and urea clearance, which narrowed the significance of changes in renal function between cases and controls.
What other hypotheses could explain the changes in renal function associated with VDA in ACKD? According to our results, the total serum calcium level was closely related to the recovery of renal function.
An increase in calcemia and calciuria stimulates the calcium-sensitive receptor located at the renal tubular level in the ascending loop of Henle, and this stimulus causes an effect similar to that of a loop diuretic (increased urinary excretion of sodium, chlorine, calcium and magnesium).35 Thus, a volume depletion due to a diuretic effect associated to an increase in calcemia could help explain the reversible decrease in glomerular filtration associated with VDA.
Another hypothesis would be related to the effect of VDA on the inhibition of the renin-angiotensin-aldosterone system (RAAS).36 While this inhibition may be useful to reduce proteinuria,1–4 it may also be responsible for a decrease in glomerular filtration rate due to the reduction of pressure both in the glomerular capillary and in the efferent capillary, which may be even more intense if the patient was on treatment with other anti-RAAS drugs or diuretics, as is the case in most of these patients. The negative effect of the double pharmacological blockade of RAAS on the deterioration and progression of CKD has already been described in other studies.37,38
The pharmacological administration of VDA causes a significant increase in FGF2339; and, elevated levels of FGF23 have been associated to a faster progression of CKD.39 Although the concentrations of FGF23 were not measured in the present study, it would be interesting to determine if FGF23 play a role in the reduction of renal function induced by VDA administration.
Finally, the hypothesis of a relationship between the control of hyperparathyroidism by itself and the decline in renal function in those treated with VDA should also be considered. This clinical relationship is well known in renal transplant patients who present significant reductions in the glomerular filtration defects when a tertiary hyperparathyroidism is reversed by parathyroidectomy.40–43 Some of this deterioration has been attributed to the prescription of VDA during the postoperative period,41 although the origin of this phenomenon is unknown so far, there is speculation about the effect of PTH on the regulation of renal perfusion and glomerular filtration.41,42
A practical consequence derived from the results of the present study is the possibility of modifying or influencing the speed of progression in the advanced stages of CKD by weighing the risks-benefits of prescribing a treatment with VDA. The discontinuation of this medication could slow the progression of advanced kidney failure and allow longer periods of survival without dialysis.
However, this study should not be taken as an allegation against the use of VDA in ACKD. These drugs are the fundamental in the control of secondary hyperparathyroidism, but their indication based on their pleiotropic effects should be based on more solid clinical evidence.
The recommendations that we dare to propose in order to prevent undesirable deterioration of kidney function in advanced CKD patients receiving VDA are: 1. To analyze and update current clinical guidelines and make more flexible the PTH target values in ACKD patients. 2. Do not start any treatment with VDA if there is no adequate control of serum phosphorus. 3. The doses of VDA at the initiation of treatment should be lower than those prescribed regularly in dialysis patients. 4. Once a treatment with VDA has been started, controls of biochemistry should be performed frequently (2–3 months), adjusting the doses according to expected response and side effects (hypercalcemia, hyperphosphoremia, etc.). 5. In case of unexpectedly rapid fall in renal function, suspend AVD and check the recovery of glomerular filtration in the short term. 6. It may also be useful to include alternative markers to creatinine as markers of glomerular filtration (e.g. cystatin C).
This study has limitations. Due to its retrospective design, firm causal relationships cannot be established. In addition to the differences already discussed between cases and controls, there could be other confounding factors not taken into account in the analysis and interpretation of the results.
In the selection process of controls there was some problem with the distribution of the etiologies of CKD. Although there was no statistically significant difference, in cases there was a greater number of polycystic kidney disease and in controls, diabetes mellitus was frequent. This disparity could be justified by the different propensity to develop secondary hyperparathyroidism – less in diabetic patient44 – and, therefore, with different probability of being treated early with VDA. However, both etiologies of CKD in their more advanced stages have a similar pattern of progression,45 so it is unlikely that this selection bias could significantly influence the evolution of renal function.
The measurement of glomerular filtration was not performed by methods of maximum reliability (clearance of exogenous substances) and, therefore, the changes observed may not be real or be influenced by uncontrolled artifacts. However, from a practical point of view, these methods of maximum reliability are not used in the usual clinic. The creatinine values and the formulas estimating the glomerular filtration derived from their serum concentration are taken as the criterion to assess the progression of kidney failure and indicate the need for dialysis. Therefore, any factor that alters the serum creatinine concentration could also influence important therapeutic decisions in these patients.
In conclusion, the discontinuation of VDA in patients with ACKD is associated with a significant and fast improvement of the estimated renal failure and slows its CKD progression. These changes are not related to urinary excretion of creatinine but to baseline serum calcium levels.
Further studies are needed to establish the mechanisms by which VDA may alter serum creatinine concentrations or truly reduce the glomerular filtration rate whose consequences in ACKD may be as serious as premature onset of dialysis.
Conflict of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Caravaca F, Caravaca-Fontán F, Azevedo L, Luna E. Cambios de la función renal tras la suspensión de análogos de vitamina D en la enfermedad renal crónica avanzada. Nefrologia. 2018;38:179–189.