Online haemodiafiltration (OL-HDF) is currently the most effective dialysis technique that also improves survival. To date, high permeability membranes with low albumin loss, such as polysulfone, polyamide and polyacrylonitrile membranes have been the most widely used. However, the initially restricted use of cellulose triacetate (CTA) membranes in OL-HDF has expanded. The aim of the study was to ascertain whether the latest generation asymmetric CTA membranes are more effective in obtaining high convective transport.
Patients and methodsA total of 16 patients (10 males and 6 females) undergoing OL-HDF were studied. Each patient underwent 4 different sessions, with haemodialysis or OL-HDF, and/or with CTA or asymmetric CTA 1.9m2 membranes. Each session was assigned in a randomised order. Serum levels of urea, creatinine, β2-microglobulin, myoglobin, prolactin, α1-microglobulin, α1-acid glycoprotein and albumin where measured at the beginning and end of each session to obtain the reduction rate. The loss of solutes and albumin was quantified from the dialysate.
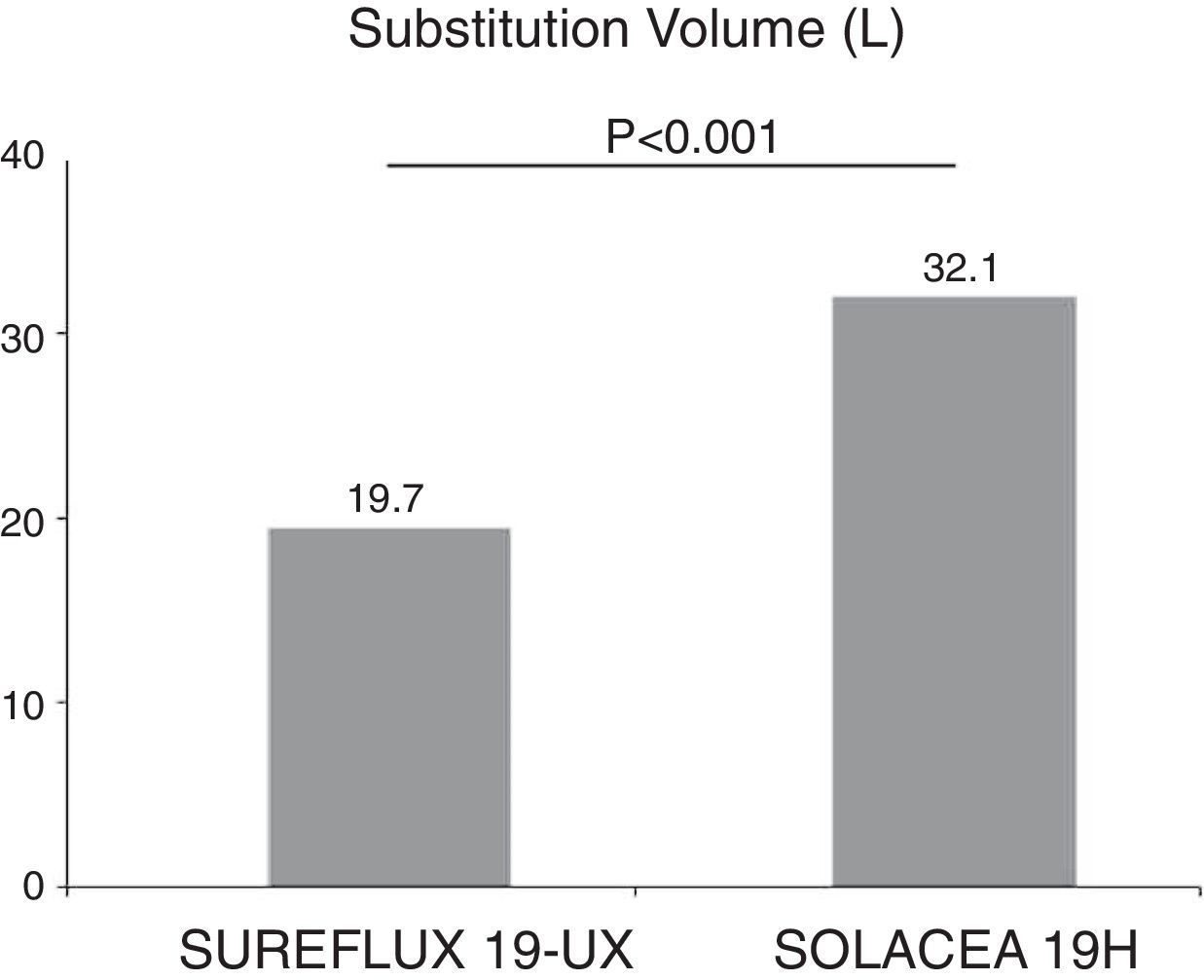
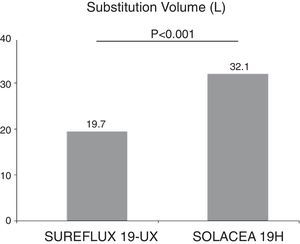
ResultsA significantly greater replacement volume in OL-HDF (32.1±3.1 vs. 19.7±4.5 l, p<0.001) was obtained by using asymmetrical CTA membranes compared to conventional CTA membranes. Regarding uraemic toxin removal, both membranes obtained similar results for small molecules, whereas asymmetric CTA membranes achieved better results for large molecules, increasing the reduction ratio by 29% for β2-microglobulin, 27.7% for myoglobin, 19.5% for prolactin, 49% for α1-microglobulin and double for α1-acid glycoprotein (p<0.01 in all situations). The loss of albumin was less than 2g for all treatment sessions.
ConclusionLatest-generation asymmetric CTA have proven to be effective in attaining OL-HDF objectives without increased albumin loss.
La hemodiafiltración on-line (HDF-OL) es actualmente la técnica de hemodiálisis (HD) más efectiva y aumenta la supervivencia. Hasta el momento presente las membranas de alta permeabilidad con baja pérdida de albúmina como las de polisulfona, poliamida y poliacrilonitrilo son las más utilizadas. Las membranas de triacetato de celulosa (TAC), limitadas inicialmente para su uso en HDF-OL, han evolucionado. El objetivo del estudio fue determinar si las membranas de nueva generación de TAC asimétrico (TACA) son más adecuadas para realizar alto transporte convectivo.
Pacientes y métodosSe estudiaron 16 pacientes, 10 hombres y 6 mujeres, en programa de HDF-OL. A cada paciente se le realizaron 4 sesiones diferentes, con HD o HDF-OL, o con filtros de TAC o TACA de 1,9m2, aleatorizando el orden. En cada sesión se determinaron concentración de urea, creatinina, β2-microglobulina, mioglobina, prolactina, α1-microglobulina, α1-glicoproteína ácida y albúmina en suero al inicio y al final de cada sesión, para calcular el porcentaje de reducción. Así mismo, se cuantificó la pérdida de solutos y albúmina en el líquido de diálisis.
ResultadosCon las membranas de TACA se consiguió un volumen de sustitución en HDF-OL significativamente superior a las membranas de TAC clásicas (32,1±3,1 vs. 19,7±4,5L; p<0,001). En términos de depuración, la eliminación de moléculas pequeñas fue similar con ambas membranas, pero, en moléculas grandes, con HDF-OL la depuración fue mayor con TACA. En HDF-OL, el porcentaje de reducción de la β2-microglobulina se incrementó un 29%, un 27,7% la mioglobina, un 19,5% la prolactina, un 49% la α1-microglobulina, y se duplicó la α1-glicoproteína ácida (p<0,01 en todas las situaciones). La pérdida de albúmina fue inferior a 2g en todas las situaciones de estudio.
ConclusiónLas membranas de TAC de nueva generación han demostrado ser eficaces para alcanzar los objetivos de HDF-OL, sin que haya una mayor pérdida de albúmina.
The number of patients receiving on-line haemodiafiltration (HDF) has increased in recent years because randomised clinical trial in prevalent patients1 have shown increased survival. Afterwards, various meta-analyses have confirmed a reduction in cardiovascular and overall mortality.2,3 Secondary analyses of studies looking at mortality as the main variable1,4,5 observed an association between convective volume and survival; so a minimum convective volume of 21l per session6 is being recommended. The main limiting factors to achieve high convective volumes are blood flow (Qb), time and the dialyser.
The pharmaceutical industry, in line with the technological advances made in haemodilaysis (HD) monitors, has developed improved dialysers able to achieve greater clearance capacity with a better adaptation to highly convective treatments. In fact, not all dialysers are appropriate to perform this type of treatment.
In a previous study7 of 11 dialysers evaluated for on-line HDF treatment, only cellulose triacetate (CTA) membranes and polymethyl methacrylate (PMMA) demonstrated to be less appropriate for on-line HDF with less β2-microglobulin (β2-m) clearance and limited capability to reach a suitable convective volume with elevated transmembrane pressure. Recently, Potier et al.8 published the use of 19 dialysers for on-line HDF; they observed that in seven of them, albumin loss per session was excessive. Therefore, the use of these dialysers for highly convective techniques is questioned.
Synthetic dialysers made of polysulfone (polyethersulfone, helixone) polyamide and polyacrylonitrile have been used most frequently in the past few years in HD and also in on-line HDF. However, in recent years, intolerance reactions (low blood pressure, desaturation) to synthetic dialysers have been observed in a small percentage of patients.9,10 This is a reason by which cellulose dialysers, such as CTA, have been reintroduced as an alternative to the dialysers previously mentioned. Initial CTA dialysers presented a low ultrafiltration coefficient, and therefore there are limitations of their use for haemodiafiltration. However, a new generation of dialysers with asymmetric CTA membranes have been designed with an increase in hydraulic permeability that deserves attention.
The aim of this study was to evaluate this new generation of asymmetric CTA dialysers (Solacea®), compared with the previous generation of CTA (Sureflux®) in HD and on-line HDF modalities.
Patients and methodsThis is an study performed in a single centre including stable HD patients. Sixteen patients were evaluated, 10 men and 6 women, with a mean age of 64.7±12 years (interval of 49–90 years) on regular HD programmes. The aetiology of chronic kidney failure was 5 chronic glomerulonephritis (31.2%), 2 diabetic nephropathies (12.5%), 1 polycystic disease (6.3%), 1 vascular nephropathy (6.3%), 2 urological causes (12.5%), 2 systemic diseases (12.5%), 1 tubular interstitial nephropathy (6.3%) and 2 undetermined aetiologies (12.5%). All patients were dialysed through an arteriovenous fistula.
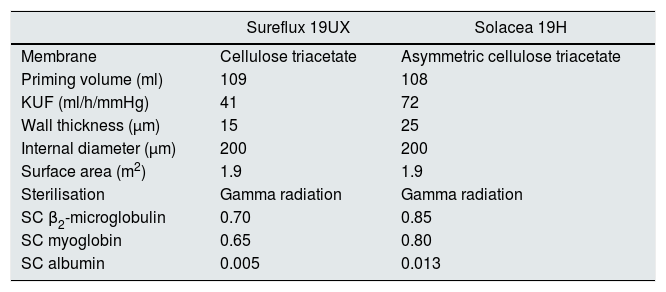
Patients were dialysed with the 5008 Fresenius monitor, suitable for on-line HDF. Four separate sessions were carried out in each patient with their usual parameters of dialysis (time 290±18min, Qb 462±34ml/min, Qd 500ml/min): 1) HD with 1.9m2 CTA (Sureflux 19 UX, Nipro), 2) on-line HDF with 1.9m2 CTA (Sureflux 19 UX, Nipro), 3) HD with new generation 1.9m2 CTA (Solacea 19H, Nipro) and 4) on-line HDF with new generation 1.9m2 CTA (Solacea 19H, Nipro). The order of the sessions was randomised. Table 1 summarises the characteristics of both dialysers.
In vitro features of the dialysers under study.
| Sureflux 19UX | Solacea 19H | |
|---|---|---|
| Membrane | Cellulose triacetate | Asymmetric cellulose triacetate |
| Priming volume (ml) | 109 | 108 |
| KUF (ml/h/mmHg) | 41 | 72 |
| Wall thickness (μm) | 15 | 25 |
| Internal diameter (μm) | 200 | 200 |
| Surface area (m2) | 1.9 | 1.9 |
| Sterilisation | Gamma radiation | Gamma radiation |
| SC β2-microglobulin | 0.70 | 0.85 |
| SC myoglobin | 0.65 | 0.80 |
| SC albumin | 0.005 | 0.013 |
KUF: ultrafiltration coefficient; SC: sieving coefficient.
Dialysers are automatically primed with the 5008 monitor. On-line HDF was performed with post-dilution infusion using an automatic infusion flow. The dialysis parameters collected in each session were: dialysis time, dialyser, dialysate flow rate (Qd), blood flow (Qb), total blood volume processed, needles, initial weight, final weight, weight gain, Kt automatically measured by ionic dialysance, recirculation index measured by the temperature module, arterial blood pressure, venous blood pressure, transmembrane pressure, initial and final haemoglobin, ultrafiltration and substitution volume.
Concentrations of urea (60Da), creatinine (113Da), β2-m (11,800Da), myoglobin (17,200Da), prolactin (23,000Da), α1-microglobulin (33,000Da) and α1-acid glycoprotein (41,000Da) and serum albumin (66,000Da) at the start (C1) and at the end (C2) of each session were determined in order to calculate the percentage reduction of these solutes (PR=100×(C1−C2)/C1). The final concentration of β2-m, myoglobin, prolactin, α1-microglobulin, α1-acid glycoprotein and albumin were corrected for haemoconcentration and volume of distribution (approximately the extracellular volume) according to Bergström and Wehle,11 according to the corrected C2 formula=C2/[1+(weight gain/0.2×final weight)]. Final extraction was done at the end of dialysis and after reducing blood flow to 100ml/min for one minute.
Urea, creatinine and albumin concentrations were measured by molecular absorption spectrometry in the ADVIA 2400 analyser (Chemistry System from Siemens Healthineers, Tarrytown, USA). β2-m and α1-acid glycoprotein were measured by immunonephelometry using the Dimension Vista (Siemens Healthineers) analyser and α1-microglobulin by immunonephelometry using the BNII (Siemens Healthineers) analyser. Myoglobin concentrations were measured by indirect enzyme immunoassay (EIA) with the Dimension EXL (Siemens Healthineers) analyser. Prolactin concentrations were measured by indirect enzyme immunoassay (EIA) with the ADVIA Centaur (Siemens Healthineers) analyser.
Dedicated reagents were used in all cases. Our laboratory's reference values are urea:10–50mg/dl; creatinine: 0.3–1.3mg/dl, β2-m: 0.1–2.3mg/l; myoglobin 0–100ng/ml; prolactin: 2.8–15ng/ml, α1-microglobulin: 5–25mg/l; α1-acid glycoprotein: 0.38–1.18g/l and albumin 34–48g/l.
Likewise, a proportional part of the dialysis fluid was collected to quantify the loss of solutes and albumin. Urea, creatinine and prolactin were measured as in serum. However, because the β2-m and albumin concentrations were very low, they had to be analysed with the same technology, but with determination methods for urine to increase analytical sensitivity. β2-m was measured with an improved detection index, 0.004625mg/l.
The results are expressed as the arithmetic mean±standard deviation. The Students’ t-test was used to analyse the statistical significance of quantitative parameters obtained from paired data, and the ANOVA test was used for repeated data. A p value of <0.05 was considered statistically significant.
ResultsWe observed a very satisfactory tolerance to these filters. No abnormal reactions were observed to the connection, disconnection, or during the on-line HD or HDF sessions. Coagulation of the lines or the dialyser did not occur in any session. Anticoagulation was low-molecular-weight heparin (tinzaparin) in 62%, heparin sodium in 19%. The remaining sessions (19%) did not include heparin.
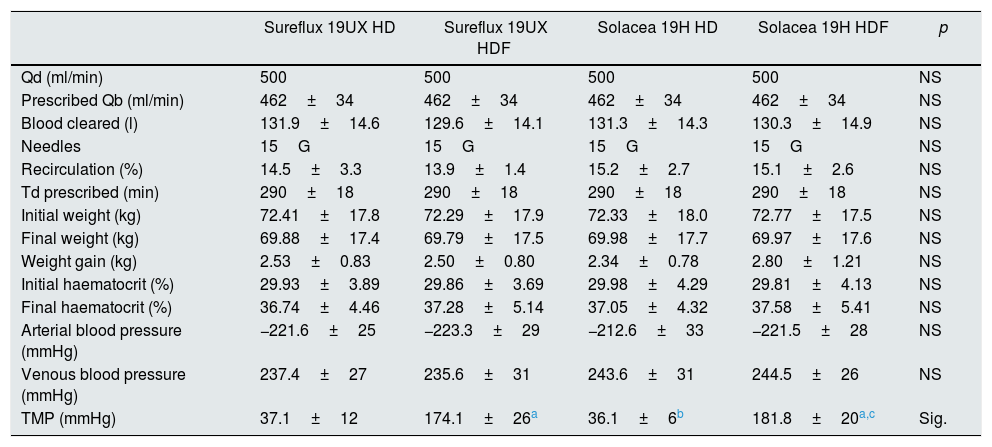
There were no differences in dialysis parameters, Qd, Qb, total blood processed by the monitor, needle size, vascular access recirculation, session duration, initial weight, final weight, weight gain, the dialysis monitor's own measurements of initial and final haematocrit, arterial blood pressure, venous blood pressure or transmembrane pressure (Table 2).
Comparison of dialysis parameters in the 4 dialyser situations (n=16).
| Sureflux 19UX HD | Sureflux 19UX HDF | Solacea 19H HD | Solacea 19H HDF | p | |
|---|---|---|---|---|---|
| Qd (ml/min) | 500 | 500 | 500 | 500 | NS |
| Prescribed Qb (ml/min) | 462±34 | 462±34 | 462±34 | 462±34 | NS |
| Blood cleared (l) | 131.9±14.6 | 129.6±14.1 | 131.3±14.3 | 130.3±14.9 | NS |
| Needles | 15G | 15G | 15G | 15G | NS |
| Recirculation (%) | 14.5±3.3 | 13.9±1.4 | 15.2±2.7 | 15.1±2.6 | NS |
| Td prescribed (min) | 290±18 | 290±18 | 290±18 | 290±18 | NS |
| Initial weight (kg) | 72.41±17.8 | 72.29±17.9 | 72.33±18.0 | 72.77±17.5 | NS |
| Final weight (kg) | 69.88±17.4 | 69.79±17.5 | 69.98±17.7 | 69.97±17.6 | NS |
| Weight gain (kg) | 2.53±0.83 | 2.50±0.80 | 2.34±0.78 | 2.80±1.21 | NS |
| Initial haematocrit (%) | 29.93±3.89 | 29.86±3.69 | 29.98±4.29 | 29.81±4.13 | NS |
| Final haematocrit (%) | 36.74±4.46 | 37.28±5.14 | 37.05±4.32 | 37.58±5.41 | NS |
| Arterial blood pressure (mmHg) | −221.6±25 | −223.3±29 | −212.6±33 | −221.5±28 | NS |
| Venous blood pressure (mmHg) | 237.4±27 | 235.6±31 | 243.6±31 | 244.5±26 | NS |
| TMP (mmHg) | 37.1±12 | 174.1±26a | 36.1±6b | 181.8±20a,c | Sig. |
Td: dialysis time; NS: not significant; Qb: blood flow; Qd: dialysate flow rate; Sig: significant; TMP: transmembrane pressure.
The post-dilution on-line HDF substitution volume was 19.7±4.5l (ranging between 18 and 34l) with Sureflux 19UX and 32.1±3.1l with Solacea 19H (interval between 26 and 37l), p<0.001 (Fig. 1). This first observation shows that this new CTA generation achieves a much higher substitution volume, and therefore it is suitable for on-line HDF.
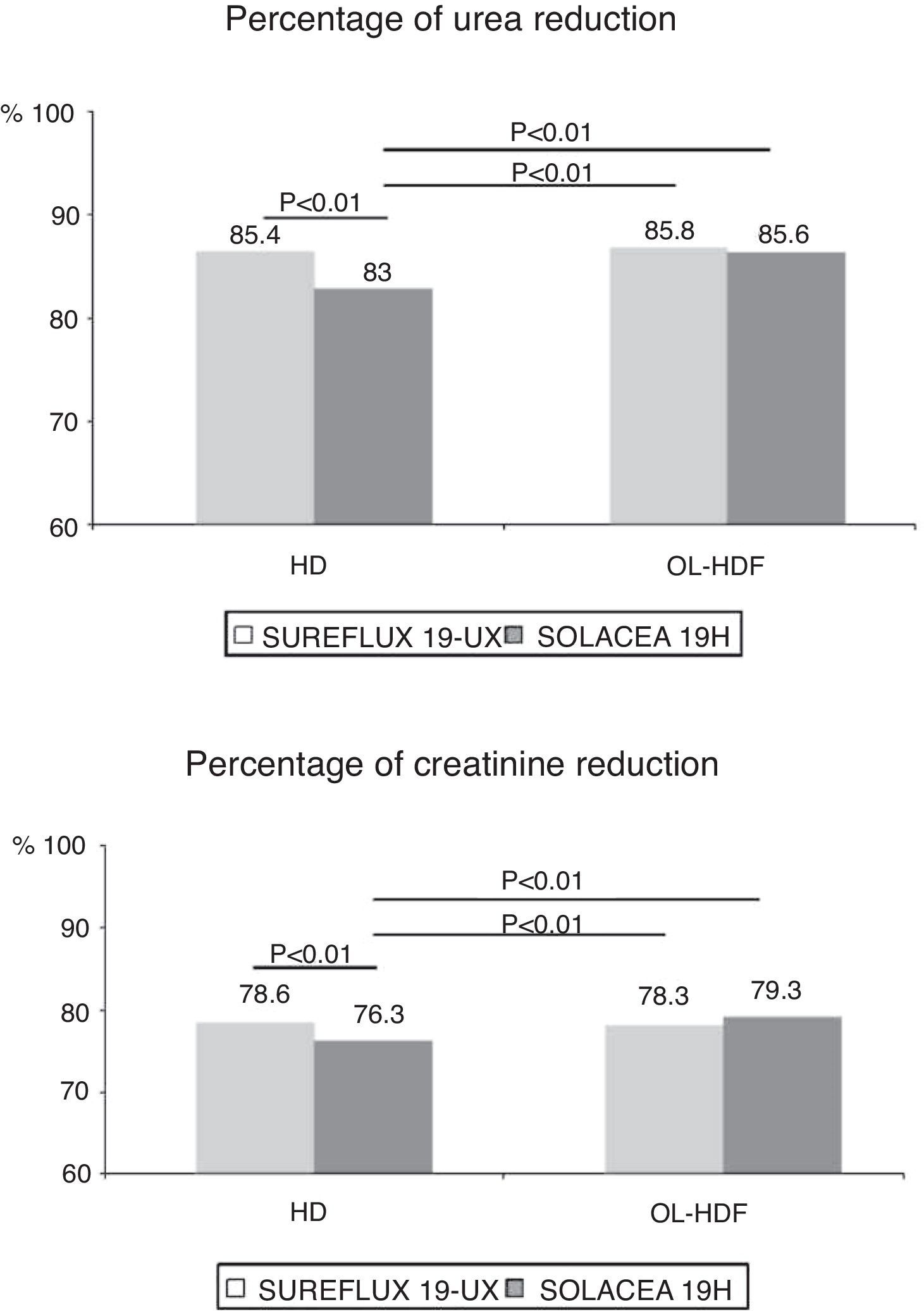
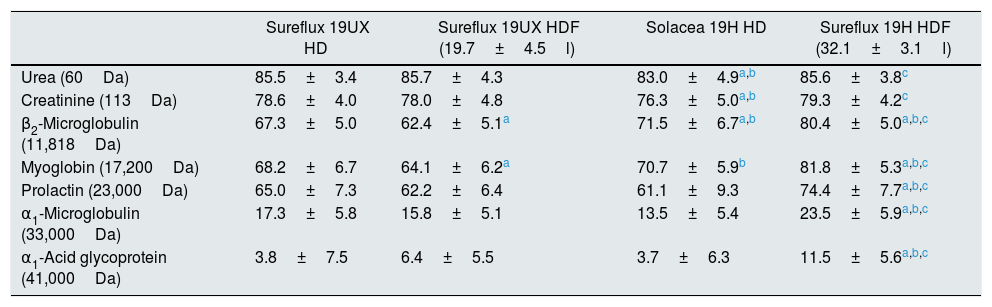
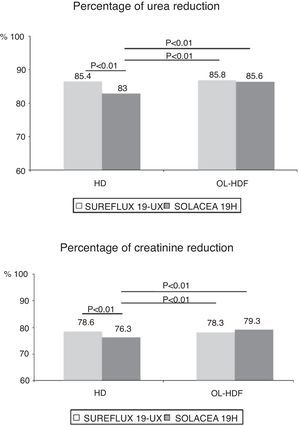
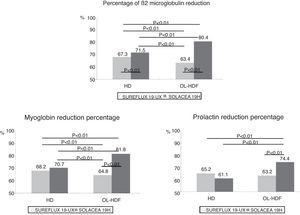
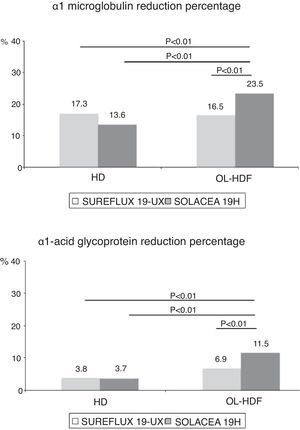
Effective clearance of solutes expressed as percentage of solute reduction is found in Table 3 and in Figs. 2–4.
Reduction percentage of solutes in haemodialysis and hemodiafiltration.
| Sureflux 19UX HD | Sureflux 19UX HDF (19.7±4.5l) | Solacea 19H HD | Sureflux 19H HDF (32.1±3.1l) | |
|---|---|---|---|---|
| Urea (60Da) | 85.5±3.4 | 85.7±4.3 | 83.0±4.9a,b | 85.6±3.8c |
| Creatinine (113Da) | 78.6±4.0 | 78.0±4.8 | 76.3±5.0a,b | 79.3±4.2c |
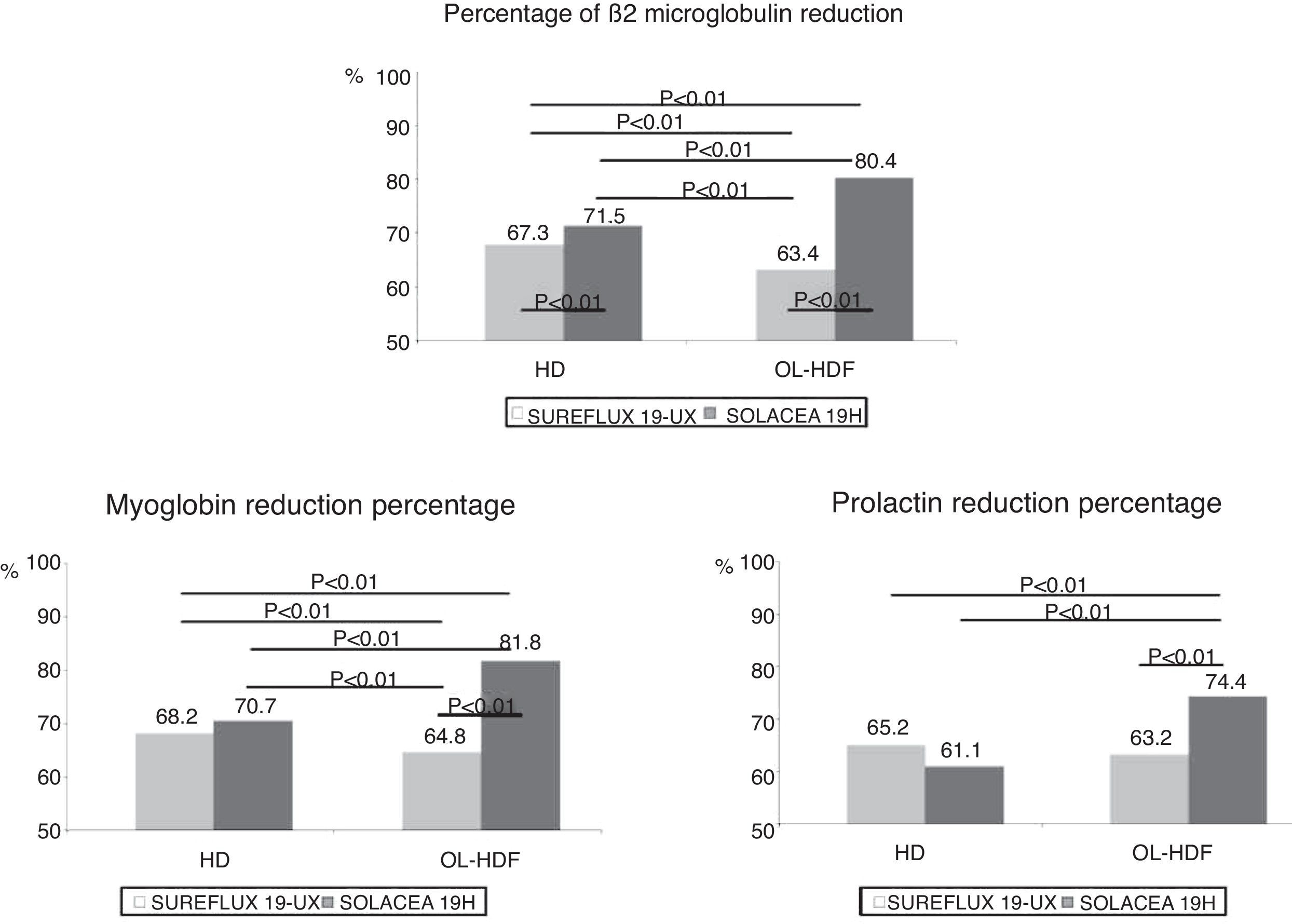
| β2-Microglobulin (11,818Da) | 67.3±5.0 | 62.4±5.1a | 71.5±6.7a,b | 80.4±5.0a,b,c |
| Myoglobin (17,200Da) | 68.2±6.7 | 64.1±6.2a | 70.7±5.9b | 81.8±5.3a,b,c |
| Prolactin (23,000Da) | 65.0±7.3 | 62.2±6.4 | 61.1±9.3 | 74.4±7.7a,b,c |
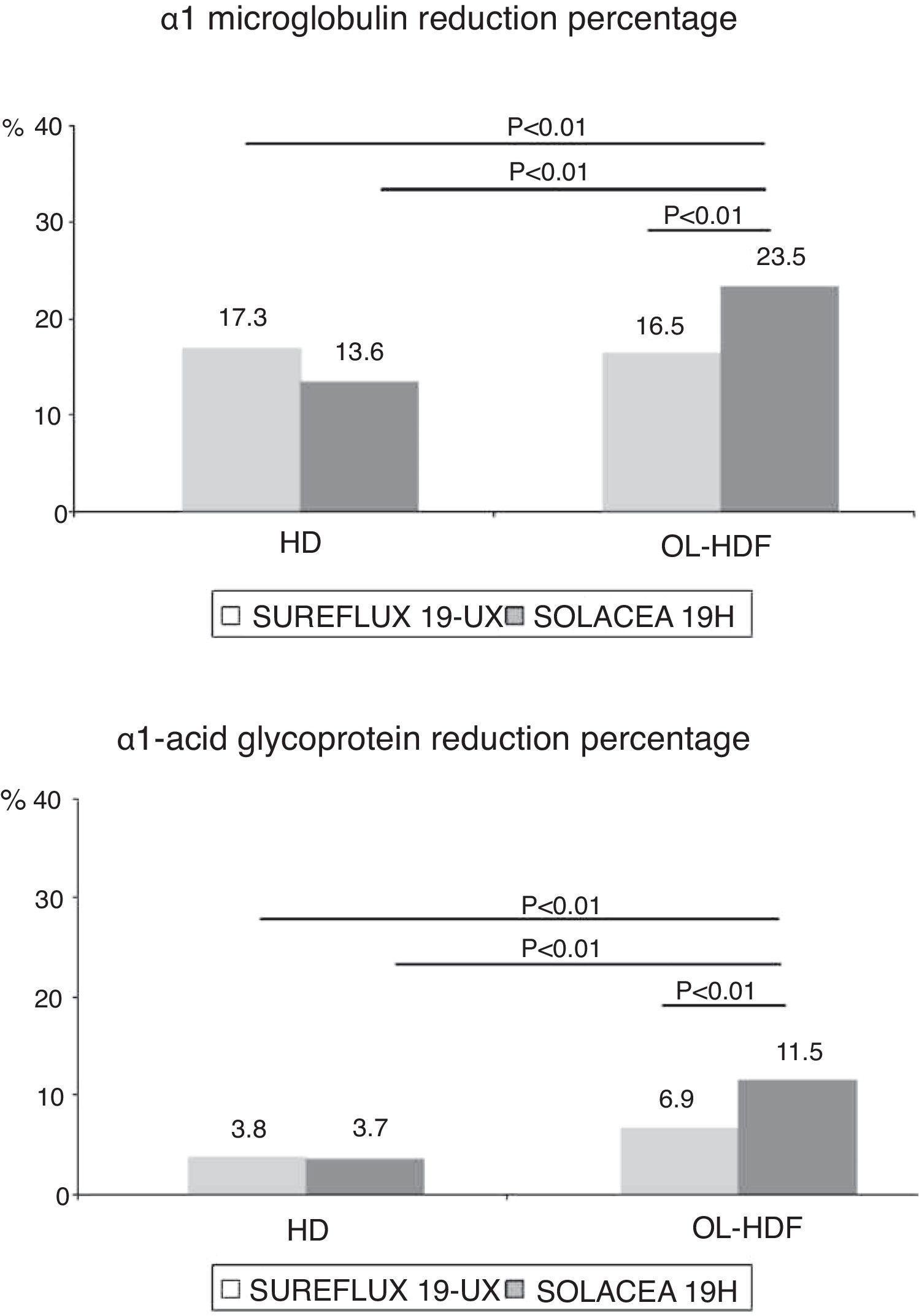
| α1-Microglobulin (33,000Da) | 17.3±5.8 | 15.8±5.1 | 13.5±5.4 | 23.5±5.9a,b,c |
| α1-Acid glycoprotein (41,000Da) | 3.8±7.5 | 6.4±5.5 | 3.7±6.3 | 11.5±5.6a,b,c |
The clearance of urea and creatinine was similar in the Solacea 19H dialyser and Sureflux 19UX in (Fig. 2).
Large moleculesIf the molecular size of solutes to be cleared is increased, there are differences observed between both filters and between the dialysis modalities. Above 30kDa, although differences between filters both in HD and in HDF are observed, the percentage reduction of these molecules is less than 25%. It therefore seems that these dialysers’ cut-off limit has already been reached. The Solacea 19H dialyser, mainly with on-line HDF, presented the greatest clearance of large molecules.
a) β2-m (11,800Da): In HD, the Solacea 19H removed 6.3% more than the Sureflux 19UX (p<0.01). In on-line HDF, the Solacea 19H removed 29% more than the Sureflux 19UX (p<0.01). Comparison of on-line HDF and HD with the same dialyser, shows that the Sureflux 19UX reduction percentage worsened by 7.3% compared to HD (p<0.01), while Solacea 19H's was 12.5% higher than with HD (p<0.01) (Fig. 3).
b) Myoglobin (17,200Da): Removal of myoglobin by the Solacea 19H dialyser is similar to that of the Sureflux 19UX dialyser in HD. However, in on-line HDF, the Solacea 19H removed 27.7% more than the Sureflux 19UX (p<0.01). Again, the Sureflux 19UX reduction percentage worsened by 6.0% as compared to HD (p<0.05), while the Solacea 19H was 15.8% higher in on-line HDF than in HD (p<0.01) (Fig. 3).
c) Prolactin (23,000Da): The Solacea 19H dialyser removes prolactin in a similar manner as the Sureflux 19UX dialyser in HD. However, in on-line HDF, the Solacea 19H removed 19.5% more than the Sureflux 19UX (p<0.01). Comparison of on-line HDF with HD with the Sureflux 19UX, shows that its reduction percentage worsened by 4.3% (NS), while with the Solacea 19H it was 21.7% higher than HD (p<0.01) (Fig. 3).
d) α1-microglobulin (33,000Da): Removal of α1-microglobulin by the Solacea 19H dialyser is similar to the Sureflux 19UX dialyser in HD. However, in on-line HDF, the Solacea 19H removed 49% more than the Sureflux 19UX (p<0.01). With Sureflux 19UX the percentage reduction was also reduced by 8.6% (p<0.01) while with the Solacea 19H it was 73% higher than in HD (p<0.01) (Fig. 4).
e) α1-acid glycoprotein (41,000Da): Removal of α1-acid glycoprotein by Solacea 19H dialyser and Sureflux 19UX dialyser in HD, are similar and both have a very limited clearance, less than 5%. However, in on-line HDF, the Solacea 19H removed almost twice as much as the Sureflux 19UX (p<0.01). Comparison of Sureflux 19UX on-line HDF to HD, shows that the percent reduction did not change, while with the Solacea 19H, it tripled (p<0.01) (Fig. 4).
It is worth to mention that that the Sureflux 19UX dialyser does not improve the clearance of large molecules with on-line HDF. In fact, it is inferior to HD (between 10,000 and 25,000Da). This performance is rather unusual and it may be assumed that even achieving 15–20l of convective volume, the clearance capacity of the pores is modified.
Albumin loss in dialysateAs in the native kidney, it is desirable a minimal loss of albumin during the dialysis sessions. The percent reduction of albumin in the blood was 6.4% with Sureflux 19UX in HD, 4.2% with Solacea 19H in HD, 5.6% with Sureflux 19UX in on-line HDF and 8.1% with Solacea 19H in HDF.
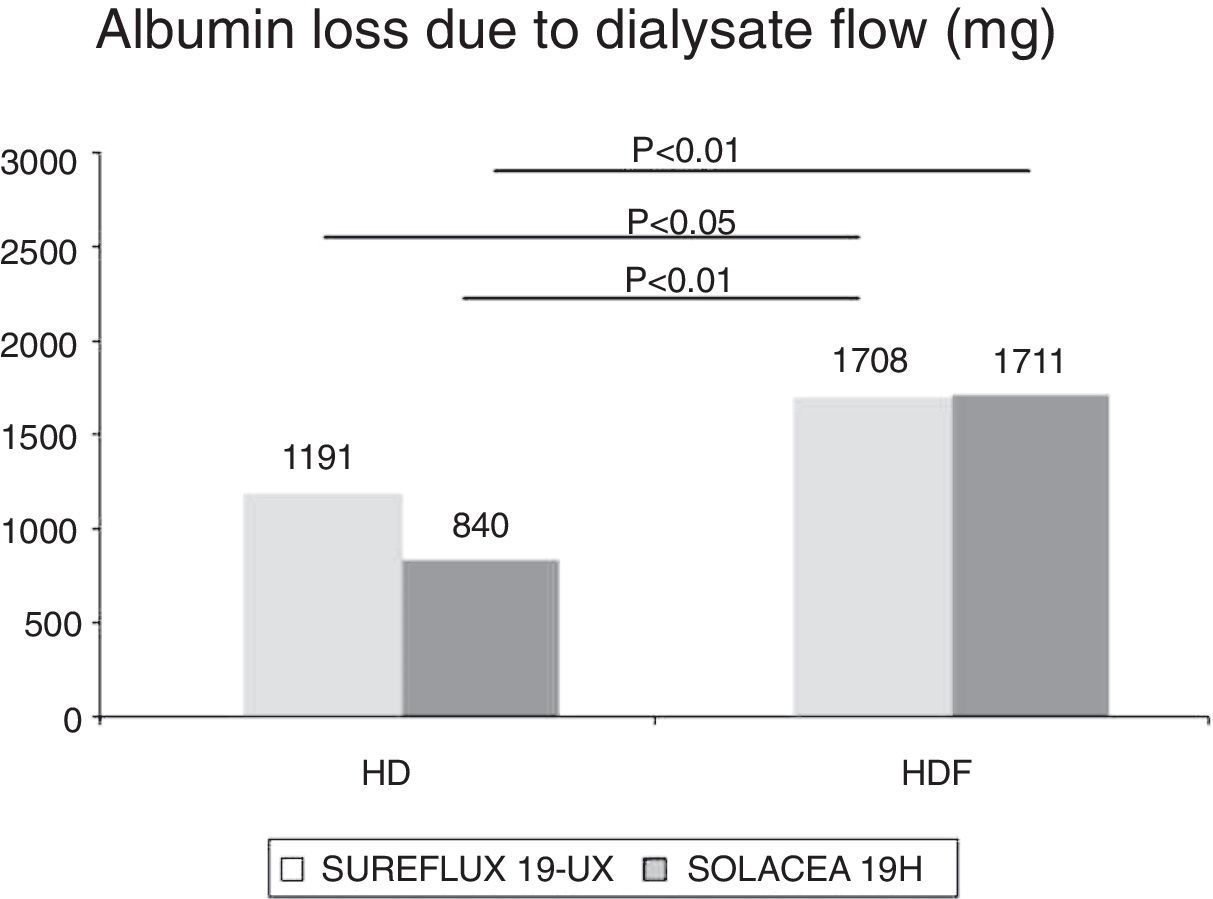
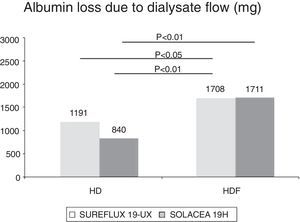
The amount of albumin eliminated in the dialysis fluid was 1.19g with Sureflux 19UX in HD, 0.84g with Solacea 19H in HD, 1.77g with Sureflux 19UX in on-line HDF and 1.71g with Solacea 19H in on-line HDF. In both HDF treatments albumin loss is significantly greater than in the other two treatments in HD (Fig. 5).
DiscussionThe results of this study demonstrate that the Solacea 19H dialyser of the new generation of asymmetric CTA dialysers shows an excellent behaviour and tolerance for both HD and on-line HDF. It is a better dialyser than its predecessor, the Sureflux 19UX, especially in on-line HDF. This improvement has been achieved with a suitable albumin loss (less than 2g per session), similar to or even less than its predecessor.
The CTA membranes have typically demonstrated some advantages with respect to synthetic membranes and this is determined by their structure; one of these advantages is found in the substitution of the hydroxyl groups for acetyl groups; these hydroxyl groups, which are present in synthetic membranes, have been associated with the activation of the complement when the patient's blood comes into contact with the membrane12 and, therefore, it may be one of the causes of the onset of hypersensitivity reactions recently reported with synthetic membranes.9,10 Another of their differentiating characteristics is that they are hydrophilic membranes. This means that they do not require the addition of hydrophiliating elements, which are another possible cause of hypersensitivity reactions.13 Additionally, CTA membranes are characterised by the presentation of a low electric charge: this will be very important in a membrane's absorption capacity because low electric charges in membranes cause a low absorption capacity,14 and this will influence the antithrombotic properties described relative to these membranes.15 Together with these properties, it is known that a low electric charge in the membrane can improve the clearance of inorganic phosphorus and the lipid profile.16 Another difference is that these membranes are bisphenol A- and polyvinylpyrrolidone-free. These are products which are present in other synthetic membranes, and which have been described as being associated with an increase in inflammation and oxidative stress.17
One of the classic limitations of cellulosic dialysers has been the low biocompatibility.18,19 Later, the changes made in their structure with the substitution of hydroxyl groups by acetyl groups have substantially improved these membranes’ biocompatibility. A clear example is the CTA modifications, which demonstrate significant improvement in their biocompatibility profiles with respect to conventional cellulosic dialysers.13 The membranes of the new asymmetric CTA generation with the modifications present in their structure could improve this profile even more compared to conventional CTAs.20
This new generation of asymmetric CTA dialysers marketed in Japan (and recently marketed in Spain) differentiates itself from the previous one in that the membrane has an asymmetric structure, an increase in capillary fibre density, and that it nearly doubles the ultrafiltration coefficient.20 This increase in membrane thickness, still less than synthetic dialysers, decreases diffusion slightly, but also allows for larger pores to be supported, increases their hydraulic permeability and, therefore, increases convective transport. Exactly as has been seen in this study, the clearance of small and large molecules in HD with asymmetric CTA is similar to that of the CTA generation, which is why a change of dialyser that continues with the HD modality would not be particularly justified. Notwithstanding, the new asymmetric CTA dialyser reached a much higher substitution volume than its CTA predecessor, with a suitable convective volume complying with current recommendations. And, more importantly, this increase in convective volume meant an increase in the clearance of all solutes analysed, especially solutes with high molecular weights. These benefits make this dialyser worth recommending both for HD use as well as its increased benefits for on-line HDF.
In corroboration with the previous study,7 the conventional CTA dialyser, Sureflux 19UX, did not provide a sufficient convective volume and, more surprisingly, this convective volume did not help to improve clearance, but instead diminished it in the case of large molecules. This is truly uncommon, since the majority of dialysers increase their clearance capacity when high convective volume is added. It seems reasonable that this dialyser should be prescribed only for HD treatments.
It is known that one of the limiting factors of dialysers, especially in on-line HDF, is the loss of albumin. In the recent analysis conducted by Potier et al.8 of 19 membranes used in on-line HDF, it is stated that some of them have a greater albumin loss than recommended, and, therefore, that they should not be used in techniques with elevated convective transport. Our study confirms the low albumin loss of these asymmetric CTA dialysers. This fact, together with its improvement in terms of mid-sized molecule clearance and its biocompatibility profile, would allow them to be used for on-line HDF.
Post-dilution on-line HDF has demonstrated to produce an increase in survival rates1 and, subsequently, various meta-analyses confirmed a reduction in cardiovascular and overall mortality.2,3 Synthetic membranes with high permeability and low albumin loss, such as polysulfones and polyethersulfones, have been used up until now to obtain a suitable convective volume and correct molecule clearance, with no evident clinical problems. In recent years, cases of hyperreactivity associated with this type of membrane have been reported.9,10 CTA membranes have been used as an alternative for dialysis in these patients and to prevent the onset of sensitivity. Up to now, it was not known whether the new asymmetric CTA filters were suitable to perform techniques with high convective transport. The data from this study show that this new generation of membranes achieve the recommended convective volume, as well as small and large molecule clearance.
We conclude that the new generation CTA dialyser, Solacea 19H, has exceeded its predecessor in all markers analysed, and would be indicated for both HD and, even more so, for on-line HDF. The prescription of the Sureflux 19UX dialyser should be limited to use in HD, because, even though on-line HDF allows for a convective volume of between 15 and 25 l, it was not associated with greater clearance of small or large molecules.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to give thanks to all patients who have participated, as well as all personnel from the Dialysis Department of the Hospital Clínic de Barcelona, for their collaboration in this study and their enthusiasm.
Please cite this article as: Maduell F, Ojeda R, Arias-Guillén M, Fontseré N, Vera M, Rodas L, et al. Una nueva generación de triacetato de celulosa adecuado para hemodiafiltración on-line. Nefrologia. 2018;38:161–168.