El receptor tipo M de la fosfolipasa A2 (PLA2R) ha sido identificado como uno de los antígenos diana de la respuesta autoinmune en la nefropatía membranosa (NM) idiopática. La prevalencia de anticuerpos anti-PLA2R en enfermos con NM idiopática oscila en torno al 70 %, pero varía en función del área geográfica y hasta la fecha no se ha demostrado que la presencia de anti-PLA2R se asocie a un determinado perfil clínico de presentación de la enfermedad. Métodos: Se estudiaron 64 adultos con síndrome nefrótico y diagnóstico de NM confirmado por biopsia renal. Cuarenta y siete pacientes presentaban NM idiopática y 17 NM secundaria. Se determinó la presencia de anticuerpos circulantes antiPLA2R por inmunofluorescencia indirecta (IFI) y su título mediante ELISA. La presencia de depósitos renales de anticuerpos anti-PLA2R se determinó mediante técnicas de inmunohistoquímica. Se calculó la sensibilidad y especificidad de las técnicas de IFI y ELISA para la identificación de los enfermos con depósitos renales y para la identificación de los enfermos con NM idiopática. Se analizó si había diferencias en el perfil clínico de la enfermedad en el momento del diagnóstico en función de la presencia o no de anticuerpos anti-PLA2R. Resultados: No se observaron diferencias significativas en las variables clínico-demográficas entre enfermos con NM idiopática y secundaria. La prevalencia de depósitos glomerulares de anti-PLA2R por IHQ fue del 76,6 %. Las técnicas de IFI y de ELISA tuvieron una sensibilidad (94,4 % IFI y 97,2 % ELISA) y una especificidad (100 %) similar para la identificación de los enfermos con depósitos renales de anti-PLA2R. La determinación de anti-PLA2R por IFI identificó a los enfermos con NM idiopática con una sensibilidad del 72,3 % y una especificidad del 94,2 %. Un título de anticuerpos > 15 RU/ml medido por ELISA tuvo una sensibilidad del 74,45 % y una especificidad del 94,2 % para la identificación de los enfermos con NM idiopática. Los pacientes con NM idiopática y anti-PLA2R presentaron cifras de proteinuria significativamente mayores (13,25 [P25-P75: 9,05-15,87] frente a 9,43 [P25-P75: 6,30-15] g/día, p: 0,018). No se apreció correlación estadística entre el título de anticuerpos medido por ELISA con la edad, el filtrado glomerular, la albuminemia y la proteinuria en 24 horas. Conclusiones: Las técnicas empleadas para la determinación de anti-PLA2R en pacientes con NM presentan alta especificidad para el diagnóstico de formas idiopáticas de la enfermedad glomerular. La frecuencia con la que se identifican pacientes con NM y anti-PLA2R es parecida a la descrita en estudios previos. La tinción por inmunohistoquímica es el método más sensible para la detección de casos de NM asociados a presencia de anticuerpos anti-PLA2R. Las técnicas de IFI y de ELISA permiten la detección de anticuerpos circulantes anti-PLA2R en la mayor parte de los enfermos con depósitos renales, pero con muy baja frecuencia pueden dar resultados falsamente negativos. La concordancia de estas pruebas es alta. Los enfermos con NM idiopática y depósitos renales de anticuerpos anti-PLA2R tienen mayor proteinuria que los enfermos anti-PLA2R negativos, pero las diferencias tienen escasa relevancia clínica.
The M-type phospholipase A2 receptor (PLA2R) has been identified as one of the target antigens of the autoimmune response in idiopathic membranous nephropathy (MN). The prevalence of anti-PLA2R antibodies in patients with idiopathic MN is around 70% but this varies in accordance with geographic region, and until present, anti-PLA2R has not been shown to be associated with any particular clinical profile of the disease. Methods: We studied 64 adults with nephrotic syndrome who were diagnosed with MN, confirmed by renal biopsy. Forty-seven patients had idiopathic MN and 17 had secondary MN. We determined the presence of circulating anti-PLA2R antibodies by indirect immunofluorescence (IIF) and their titre by ELISA, and we analysed the presence of anti-PLA2R antibody renal deposits by immunohistochemical techniques. We calculated the sensitivity and specificity of the IIF and ELISA techniques for the identification of patients with renal deposits and for the identification of those with idiopathic MN and we tested whether there were differences in the clinical profile of the disease at the time of diagnosis according to the presence or absence of anti-PLA2R antibodies. Results: We did not observe significant differences in the clinical-demographic variables between patients with idiopathic and secondary MN. The prevalence of anti-PLA2R glomerular deposits by IHC was 76.6%. The IIF and ELISA techniques had a similar sensitivity (IIF 94.4% and ELISA 97.2%) and specificity (100%) for the identification of patients with anti-PLA2R renal deposits and the detection of circulating anti-PLA2R antibodies. The determination of anti-PLA2R by IIF identified patients with idiopathic MN with a sensitivity of 72.3% and a specificity of 94.2%. A titre of antibodies >15RU/ml measured by ELISA had a sensitivity of 74.45% and a specificity of 94.2% for the identification of patients with idiopathic MN. Patients with idiopathic MN and anti-PLA2R had significantly higher proteinuria figures (13.25 [P25-P75: 9.05-15.87] compared to 9.43 [P25-P75: 6.30-15] g/day, P:.018). No statistical correlation was observed between the antibody titre measured by ELISA and age, glomerular filtration rate or 24-hour proteinuria or albuminaemia. Conclusions: The techniques employed to determine anti-PLA2R in patients with MN are highly specific for the diagnosis of idiopathic forms of the glomerular disease. The frequency with which patients with MN and anti-PLA2R were identified is similar to that reported in previous studies. Staining by immunohistochemistry is the most sensitive method for detecting cases of MN associated with the presence of anti-PLA2R antibodies. The IIF and ELISA techniques allow circulating anti-PLA2R antibodies to be detected in most patients with renal deposits, but they may very infrequently have false negative results. The concordance of these tests is high. Patients with idiopathic MN and anti-PLA2R antibody renal deposits have higher proteinuria than patients that are anti-PLA2R negative, but the differences have little clinical importance.
INTRODUCTION
Membranous nephropathy (MN) is the main cause of idiopathic nephrotic syndrome in adults.1 Its pathophysiological basis is the formation of immune deposits in the subepithelial space, between the lamina rara externa of the glomerular basement membrane and the podocyte.2 MN is classified as idiopathic or secondary depending on whether or not it is possible to find an aetiology responsible for it. In the absence of a disease that is systemic, infectious, neoplastic or the result of exposure to drugs, distinguishing between the two forms is often complicated and cannot be carried out using only renal biopsy data. Most evidence available indicates that in idiopathic forms, renal deposits form in situ after antibodies bind to target podocyte antigens,3 while secondary forms occur either as a result of the subepithelial deposit of circulating immune complexes that contain extrarenal antigens or as a result of the formation of antibodies against extrarenal antigens that bind to the basement membrane’s external surface.4 The M-type phospholipase A2 receptor (R) (PLA2R) has recently been identified and is present in the podocyte membrane, as one of the target antigens of the autoimmune response in idiopathic MN5 and it has been reported that mostly circulating IgG4 antibodies against it (anti-PLA2R) are significantly correlated with the disease’s clinical activity6 and with response to treatment.7,8 Using immunofluorescence or immunohistochemistry techniques, it is possible to demonstrate the presence of anti-PLA2R antibody deposits in the kidney. The report of these antibodies has generated great expectations in terms of their usefulness for identifying idiopathic MN subgroups with different prognoses or clinical profiles and the possibility of distinguishing between different idiopathic and secondary forms. The evidence available indicates that anti-PLA2R antibody prevalence in idiopathic MN patients fluctuates around 70%,5,9 but, to date, it has not been demonstrated that the presence of anti-PLA2R is associated with any particular clinical profile of the disease. Although it has been reported that these antibodies are highly specific to idiopathic MN, the frequency that potential secondary aetiologies are detected in anti-PLA2R-positive patients, reported in different studies, is variable.9-16 A high correlation has been reported between circulating levels and evidence of renal deposits; however, the presence of anti-PLA2R antibody deposits has been observed in patients whose circulating antibody level is negative.17,18 Differences between studies could be due to ethic-geographic reasons, but they could also be related to the type of technique used for detection (given that detection techniques used in studies published have been diverse and non-standardised) and to whether or not the level of circulating antibodies (ab) and the presence of renal deposits were simultaneously analysed.
The objectives of this study are:
To analyse the prevalence of idiopathic MN patients with high levels of circulating anti-PLA2R ab using immunofluorescence and ELISA techniques.
To analyse the concordance between circulating levels and renal deposits of anti-PLA2R antibodies.
To analyse the usefulness of circulating levels and renal deposits of anti-PLA2R for distinguishing between idiopathic and secondary forms of MN.
To analyse whether in idiopathic MN patients there is any difference in the baseline clinical profile in accordance with initial anti-PLA2R positivity.
PATIENTS AND METHOD
We studied a sample of 64 patients older than 18 years of age, with the following criteria: nephrotic syndrome and diagnosis of MN confirmed by renal biopsy. Forty-seven of them had idiopathic MN and 17 had secondary MN. MN was diagnosed whenever there was a compatible morphological pattern, along with evidence of subepithelial IgG and C3 deposits in the immunofluorescence and electron microscope. After a study with protocol (which, as well as a complete anamnesis and physical examination, included autoimmunity-related serological studies, renal and urinary tract ultrasounds, chest x-rays and CT scans of the chest and abdomen in all cases, followed by endoscopic studies according to initial findings), patients in whom the aetiology was not identified were classified as idiopathic MN and those in whom a cause could be identified were classified as secondary MN patients (one with colon adenocarcinoma, one with clear cell renal cell adenocarcinoma, three with autoimmune thyroiditis, eight with lupus nephritis type V, two with mixed connective tissue disease and two secondary to treatment with non-steroidal anti-inflammatory drugs). At the time of diagnosis, we measured total serum cholesterol, serum creatinine, serum albumin, 24-hour proteinuria, estimated glomerular filtration rate using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula,19 anti-PLA2R ab levels by indirect immunofluorescence (IIF) (Anti-Phospholipase A2 receptor IIFT; Euroimmun AG, Lübeck, Germany)20 and by ELISA (Euroimmun, Lübeck, Germany; linearity: 6-1500RU/ml; lower detection limit 0.6RU/ml).
The renal biopsies were stained with haematoxylin and eosin, PAS-methenamine silver and Masson's trichrome for morphological analysis and we performed immunofluorescence studies with ab against IgA, IgG, IgM, C3, fibrinogen and light chains. The immunohistochemistry procedures for detecting anti-PLA2R ab were performed in paraffin-embedded tissue after deparaffinisation and rehydration, in accordance with the avidin-biotin-peroxidase method, using anti-PLA2R ab (HPA012657 Sigma-Aldrich Co.LLC).
This study adhered to the parameters established by the Declaration of Helsinki. All patients gave their informed consent in writing and the centre’s bioethical committee approved the study.
Statistical analysis
The results are expressed as means and standard deviation for normally distributed variables or as medians and quartiles for non-normally distributed variables. The differences between group means were analysed using the Student’s t-test for independent data or the Mann-Whitney U test. The differences in proportions were analysed using the χ2 test or Fisher's exact test. We calculated sensitivity and specificity of anti-PLA2R by IIF and the presence of renal deposits of anti-PLA2R ab for the diagnosis of idiopathic MN. Likewise, using ROC curves, we determined the anti-PLA2R ab titre, measured by ELISA, with the greatest sensitivity and specificity for identifying patients with renal deposits of anti-PLA2R ab. Once determined, we calculated the sensitivity and specificity of the aforementioned value for the diagnosis of idiopathic MN. All P values <.05 were considered statistically significant. We used the SPSS version 20.0 statistical software.
RESULTS
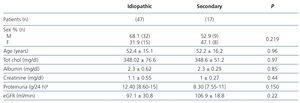
Table 1 summarises the clinical-demographic characteristics of patients studied with MN. We did not observe significant differences with regard to sex, age, total cholesterol, proteinuria, serum albumin and glomerular filtration rate between patients with idiopathic and secondary MN.
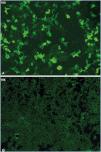
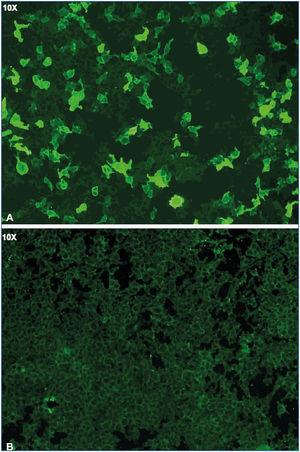
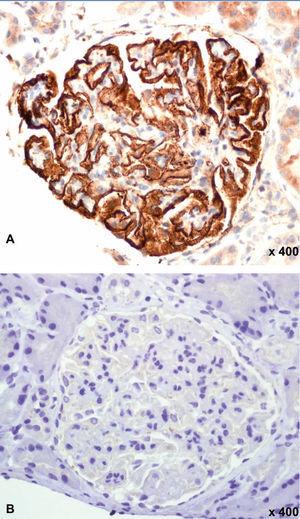
Figure 1 displays images representing the positivity and negativity of the IIF study for circulating anti-PLA2R ab detection and Figure 2 displays images representing immunohistochemical studies with and without evidence of anti-PLA2R ab renal deposits.
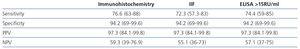
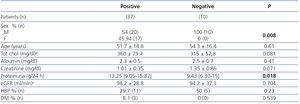
The prevalence of anti-PLA2R ab renal deposits by IHC was 76.6% in patients with idiopathic MN compared to 5.8% in those with secondary MN (P: 0.0001). The IIF had a sensitivity of 94.4% and specificity of 100% for identifying patients with renal deposits, and a sensitivity of 74.4% and specificity of 94.1% for identifying patients with idiopathic MN (Table 2). In ELISA, the median ab titre at the time of diagnosis was 42.41RU/ml (P25-75: 17-90RU/ml) in patients with idiopathic MN and 12RU/ml (P25-75: 0-13.4RU/ml) in patients with secondary MN (P: 0.0026). Patients with idiopathic MN and evidence of anti-PLA2R ab renal deposits had a significantly higher ab titre than patients with idiopathic MN without ab deposits in the biopsy (43.8RU/ml [P25-75: 16-100RU/ml] versus 1.23RU/ml [P25-75: 0-3.8RU/ml], P: 0.00014). Using ROC curves, we observed that, in ELISA, an anti-PLA2R ab titre >15RU/ml was the most sensitive (94.5%) and specific (100%) value for identifying patients with anti-PLA2R ab renal deposits, and as such, this value was taken as a cut-off for considering an ELISA result as positive. Applying these criteria to the sample studied, ELISA had a sensitivity of 74.4% and specificity of 94.2% for the diagnosis of idiopathic MN (Table 2). We only found evidence of detectable anti-PLA2R levels and renal deposits using IIF and ELISA in 1 (5.8%) of the 17 patients classified as secondary MN patients. After carrying out examinations with protocol to rule out secondary aetiologies, we diagnosed this patient with clear cell renal cell adenocarcinoma. Two patients with anti-PLA2R ab renal deposits were negative in IIF and one of them was also negative in ELISA. Both, at the time when samples were taken, had nephrotic syndrome with mean proteinuria levels of 12±2.6g/day. The ELISA titres were 13.2 and 15.4RU/ml, respectively. Table 3 summarises the clinical-demographic characteristics of idiopathic MN patients in accordance with the presence or absence of anti-PLA2R ab in IHC. The patient group with idiopathic MN and negative anti-PLA2R ab consisted solely of males. Patients with positive anti-PLA2R had significantly higher proteinuria figures (13.25 [P25-P75: 9.05-15.87] versus 9.43 [P25-P75: 6.30-15] g/day, p: 0.018), but we did not observe significant differences in any of the other variables studied. We did not observe a statistical correlation between the ab titre measured by ELISA and age, renal function, serum albumin level or 24-hour proteinuria.
DISCUSSION
The results of our study indicate that 76.6% of idiopathic MN patients have anti-PLA2R ab renal deposits that are detectable by IHC. The presence of these deposits is associated with circulating levels of anti-PLA2R ab, whose presence may be demonstrated in 94.4% of cases using IIF techniques and in 97.2% of cases using ELISA. The prevalence of circulating ab with both techniques is similar to that described in previous studies that used IIF,20 ELISA21 and Western Blot5 techniques.
The first aspect of interest in terms of techniques for detecting circulating anti-PLA2R ab is their sensitivity in the identification of patients in whom there is evidence of renal deposits of the former. In this regard, in our experience, ELISA and IIF techniques have similar results. All patients with circulating ab levels detected using one or another of the techniques had anti-PLA2R renal deposits. However, it was possible to demonstrate the presence of anti-PLA2R ab renal deposits in the absence of detectable circulating levels. This evidence is consistent with data previously reported by other groups17,18 and could be explained by a lack of parallelism between the evolution of circulating ab titres and that of anti-PLA2R renal deposits. In our experience, patients in whom renal deposits were detected, but not circulating anti-PLA2R ab, had titres measured by ELISA that were close to the cut-off value defined for categorising the groups and, in all cases, they displayed unquestionable criteria of nephrotic syndrome without clinical differences compared to patients with circulating levels of anti-PLA2R. Given the great difference between the ab titre in these cases and that detected in MN forms in which there was no evidence of anti-PLA2R renal deposits (values around 0), our data suggest that whenever circulating levels of anti-PLA2R are not detected by IIF techniques, although the ELISA result is also negative, if titres detected by the latter are close to 15RU/ml, it is possible that there will be anti-PLA2R ab subepithelial deposits in the renal biopsy.
The second aspect of interest in terms of circulating anti-PLA2R ab detection techniques is their clinical value for distinguishing between idiopathic and secondary MN. IIF techniques, being qualitative, may lead to results that are difficult to interpret or falsely negative at low ab titres. ELISA techniques require a cut-off point to be defined from which the result obtained is considered positive. In our experience, the most sensitive and specific titre for detecting patients with anti-PLA2R ab renal deposits was 15RU/ml, a value that is slightly lower than the cut-off point defined by the laboratory that developed the measurement system. This value had a similar sensitivity to that of the IIF, and as such, it should be concluded that both techniques are comparable. The advantages of one or the other would depend on some of their distinguishing characteristics. ELISA techniques allow circulating ab to be detected and quantified. As such, they would theoretically be applicable in cases in which the aim is to monitor the evolution of titres in prospective follow-ups, although their clinical use for this purpose has not yet been formally analysed. However, ELISA techniques currently available have a high cost, their processing is slow and they cannot be applied to the analysis of individual samples, while IIF techniques, since they allow the analysis of small sample groups, may provide a qualitative or semi-quantitative result in a shorter time period.
After identifying anti-PLA2R ab as potential mediators of renal lesions in idiopathic MN, there were expectations with regard to the possibility that their detection would allow the diagnosis of secondary MN and particularly associated neoplasia to be ruled out. From their description, most studies indicate that the detection of renal deposits or circulating anti-PLA2R ab is highly specific to idiopathic MN. However, there are certain discrepancies between the different studies in terms of the percentage of patients with positive anti-PLA2R ab in whom a potential responsible aetiology was detected.9 Few cases are reported in which MN with anti-PLA2R ab is associated with a systemic or infectious disease.9,11,20 Our data are in line with those of previous studies5,11,13,18 in which the presence of anti-PLA2R ab is highly specific to the diagnosis of idiopathic MN. In our experience, in no patient with MN associated with a systemic disease or drugs could we demonstrate the presence of renal deposits or circulating anti-PLA2R ab, but one of the two patients in whom MN was associated with neoplasia displayed renal deposits and circulating ab levels. As such, in spite of the anti-PLA2R ab association with neoplasia reported in different studies being very low9,11 and that in some cases it could be a coincidental finding, until we have more experience, it would not be wise to perform studies with the purpose of ruling out potential secondary forms in patients with MN and evidence of anti-PLA2R ab. The last objective proposed in this study was to analyse whether in patients with idiopathic MN there was any type of difference in the disease’s clinical profile in accordance with the presence or absence of anti-PLA2R ab. In this regard, our results are consistent with previous studies in which patients with positive anti-PLA2R have a significantly higher level of proteinuria (but with little clinical relevance), at the time of diagnosis, but no significant differences in any of the other variables analysed.
In summary, our data indicate that after the diagnosis of MN by renal biopsy, staining by immunohistochemistry is the preferable technique for locating cases that are associated with the presence of anti-PLA2R antibodies. The IIF and ELISA techniques allow circulating anti-PLA2R ab to be detected in most patients with renal deposits, and as such, as some authors suggest,9 their use could be considered as non-invasive screening in patients with nephrotic syndrome. Nevertheless, we must bear in mind that, although it is very uncommon, they may give falsely negative results. When used for categorising patients in idiopathic and secondary forms, both techniques have comparable results. ELISA techniques have the additional value of allowing ab titres to be quantified in a simple manner. As such, they could be preferable or complementary to IIF if there is a relationship between the ab titre and the spontaneous clinical course of the disease, the response to treatment and/or the prediction of recurrence.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Baseline clinical-demographic variables of the sample.
Table 2. Sensitivity, specificity and predictive values of each test for the diagnosis of idiopathic membranous nephropathy.
Table 3. Clinical and demographic characteristics according to the presence of anti-PLA2R deposits in idiopathic membranous nephropathy patients.
Figure 1. Indirect immunofluorescence for anti-PLA2R in serum.
Figure 2. Immunohistochemistry for anti-PLA2R in a renal biopsy.