Polycystic kidney disease is a clinical and genetically heterogeneous disorder. The autosomalrecessive polycystic kidney disease (ARPKD) is less common than the autosomal dominant variant (ADPKD); the incidence rate is approximately 1 in every 20,000 births. The causal gene is called PKHD1 (polycystic kidney and hepatic disease 1). It encodes the protein fibrocystin that plays a key role in the differentiation of the renal tubules and the bile ducts.
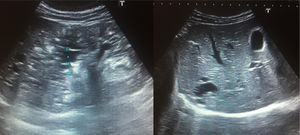
We have had the opportunity to study a family with ARPKD with unusual characteristics. The proband is currently 15 years old; when he was two months of age he was diagnosed with chronic kidney disease and liver cysts. Since he was four years old it was observed a defect in the ability to maintain mental concentration. The current GFR is 56.2mL/min/1.73m2. He has secondary arterial hypertension and liver involvement with cysts in segment 6 (Fig. 1). His 12-year-old sister is asymptomatic and the kidney and liver ultrasound is normal; renal function is preserved (GFR 113mL/min/1.73m2). Both siblings carry two compound heterozygous mutations in the PKHD1 gene, p.Arg1624Trp and p.Ile2957Thr. The mother is a carrier of the p.Ile2957Thr mutation and the father of p.Arg1624Trp.
As a hereditary family history, in the paternal branch, the grandfather was diagnosed with renal cysts without apparent alteration in glomerular filtration, the grandmother died due to a brain aneurysm without further work up to comment and a great-uncle died with cystic kidney disease. In the maternal branch, there are only mention of two aunts with recurrent urinary infections one of them with hypercalciuria. Consanguinity is denied.
In studies performed on the PKHD1 gene in patients with ARPKD, it has been observed that the p.Arg1624Trp mutation is one of the four most frequently found. Carrier patients have clinical manifestations since the neonatal period1,2 and show chronic kidney disease, renal hypodysplasia, and liver involvement.3
The second mutation, p.Ile2957Thr, has been described in consanguineous families. Likewise, it has also been classified as pathological since an early age in Caucasian races.4,5
There are genomic studies that have reported gene variants in asymptomatic patients.6 However, they consist of silent exonic changes that do not alter the amino acid sequence. None of these variants corresponds to those found in the family that we are reporting.
Explaining the phenotypic variability in both siblings is complex. The proband did not present his first symptoms in the immediate prenatal or postnatal period, as described in the majority of ARPKD cases. Presently the patient is at stage 3 of KDIGO classification, at an age that most patients who have survived would require renal replacement therapy.7 Obviously, in her sister the signs of the disease could appear late, a reason to maintain her under surveillance. It is possible that in this family there is an active DNA methylation process as has been described in the autosomal dominant variant.8
Please cite this article as: Luis-Yanes MI, Martínez Gómez G, Tapia-Romero C, Tejera-Carreño P, García-Nieto VM. Presencia de mutaciones en heterocigosis compuesta en el gen PHKD1 en una paciente asintomática. Nefrologia. 2020;40:672–673.