Linagliptin does not require dose adjustment in diabetes mellitus patients with chronic kidney disease (CKD). But, renal effects of linagliptin are not clear. Our aim was to examine the effect of linagliptin on renal disease progression in only insulin dependent type 2 diabetes mellitus (DM) patients with CKD.
MethodsStage 3–4 CKD patients were randomized into 2 groups in this prospective randomized controlled study. In the first group, linagliptin 5mg was added in addition to the background insulin therapy. In the second group, patients continued their insulin therapy. Patients were followed up at 3-month intervals for one year.
ResultsThe study population consisted of 164 patients (90 patients in linagliptin group, 74 patients in other group) with a mean age of 67.5±8.8 years. eGFR significantly increased in linagliptin group (p=0.033), but decreased in other group (p=0.003). No significant change was observed in total insulin dose in linagliptin group (p=0.111), but in other group, total insulin dose significantly increased (p<0.001). Proteinuria levels decreased in both groups, but there was no significant change. In the multiple logistic regression analysis, male gender and proteinuria emerged as variables that showed significant association with increased risk and the use of linagliptin emerged as variable that showed significant association with decreased risk for CKD progression.
ConclusionLinagliptin in DM patients with CKD was able to improve renal progression without significant effect on proteinuria and glucose control. With regard to treating diabetic nephropathy, linagliptin may offer a new therapeutic approach.
La linagliptina no precisa un ajuste de la dosis en pacientes con diabetes mellitus y enfermedad renal crónica (ERC). No obstante, los efectos renales de la linagliptina no están claros. Nuestro objetivo fue examinar el efecto de la linagliptina en la evolución de la enfermedad renal únicamente en pacientes con diabetes mellitus de tipo 2 insulinodependientes con ERC.
MétodosEn este estudio prospectivo, aleatorizado y controlado, se asignaron de forma aleatoria pacientes con ERC en estadios 3-4 en 2 grupos. En el primer grupo se añadió linagliptina 5mg además de la insulinoterapia de base. En el segundo grupo, los pacientes siguieron con su insulinoterapia. Los pacientes fueron objeto de seguimiento a intervalos de 3 meses durante un año.
ResultadosLa población del estudio estuvo compuesta por 164 pacientes (90 pacientes en el grupo de linagliptina, 74 pacientes en el otro grupo) con una edad media de 67,5±8,8 años. La TFGe aumentó significativamente en el grupo de linagliptina (p=0,033), pero disminuyó en el otro grupo (p=0,003). No se observó ningún cambio significativo en la dosis total de insulina en el grupo de la linagliptina (p=0,111), pero, en el otro grupo, la dosis total de insulina aumentó significativamente (p<0,001). Los niveles de proteinuria disminuyeron en ambos grupos, pero no hubo cambios significativos. En el análisis de regresión logística múltiple, el género masculino y la proteinuria destacaron como variables que mostraban una asociación significativa con el aumento del riesgo y el uso de la linagliptina destacó como variable con una asociación significativa con la disminución del riesgo de progresión de la enfermedad renal crónica.
ConclusiónLa linagliptina en pacientes con DM y ERC consiguió mejorar la evolución renal sin un efecto significativo sobre la proteinuria y el control glucémico. En lo que respecta al tratamiento de la nefropatía diabética, la linagliptina puede ofrecer un nuevo enfoque terapéutico.
Chronic kidney disease (CKD) is increasingly recognized as a global public health problem.1 Type 2 diabetes mellitus (DM) is one of the most common cause of CKD.2 Furthermore, DM contribute to the progression of CKD like other risk factors including, dyslipidemia, ischemia, infection, toxins, and autoimmune and inflammatory diseases.3 In this regard, DM holds therapeutic promise as a potential modifiable risk factor for CKD.
Dipeptidyl peptidase (DPP)-4 inhibitors exert beneficial effects on renal morphology and function in rodent diabetes models4–8 and some of these renal protection effects are independent from their glucose-lowering effects.9 There is a broad range of substrates for the DPP-4 enzyme including brain natriuretic peptide, substance P, peptide YY, neuropeptide Y, and stromal cell-derived factor-1 alpha, which are thought to contribute to beneficial renal effects10,11; however, the underlying mechanisms have not yet been fully elucidated. In diabetic mouse models, Takashima et al.’s study suggested that renal stromal cell-derived factor-1 upregulation by DPP-4 inhibition produces multiple protective actions on the diabetic kidney.12
Linagliptin is a selective DPP-4 inhibitor and preliminary clinical data demonstrated that linagliptin had glucose-lowering efficacy and hypothesized potential kidney benefits.13,14 Linagliptin can lower albuminuria on top of the recommended standard treatment in patients with type 2 DM.14 Tsuprykov et al showed that linagliptin delays renal disease progression in a nondiabetic, nonglucose- dependent rodent CKD model.15 DPP-4 inhibition with linagliptin may therefore be a novel approach for the treatment of CKD in general.
Although no dose adjustment is required for patients with renal impairment, clinical study experience with linagliptin in patients with CKD is limited. Most of the studies showing the effects of linagliptin on renal progression were experimental studies on models of diabetic nephropathy.9,11,16 The potential beneficial effects of DPP-4 inhibitors, including linagliptin, in preventing and treating progression of kidney disease in patients with type 2 DM is supported by retrospective analyses of clinical trials.14,17 Therefore, the clinical implications of renoprotective effects of linagliptin in experimental studies are not clear. Our aim was to examine the effect of linagliptin on renal disease progression in only insulin dependent type 2 DM patients with CKD. Our study was the first to investigate effect of linagliptin on renal progression in only advanced stage of CKD patients.
Materials and methodsStudy design and participantsThis was a prospective randomized controlled study involving stage 3–4 CKD patients who had estimated glomerular filtration rate (eGFR) lower than 60mL/min and who were referred to the Nephrology and Internal Medicine Outpatient Unit at the Kayseri Territary Care Research Hospital, Turkey, between March and September 2017. Study participants were followed until September 2018. Patients were eligible for study if they were aged 18–80 years, had type 2 DM and had hemoglobin A1c (HbA1c) values of greater than 6.5%. Participants with end-stage renal disease, defined as an eGFR less than 15mL/min/1.73m2 or requiring maintenance dialysis, were excluded. Moreover, patients with heart failure, nephrotic syndrome, chronic inflammatory diseases, or cancer were also excluded from the study. All patients were provided with information on CKD care with particular emphasis on dietary salt restriction, nephrotoxin avoidance, and strict blood pressure (BP) control. The study was performed in accordance with the Helsinki Declaration and approved by the local Ethics Committee of Erciyes University Medical School. In addition, written informed consent was obtained from all study patients.
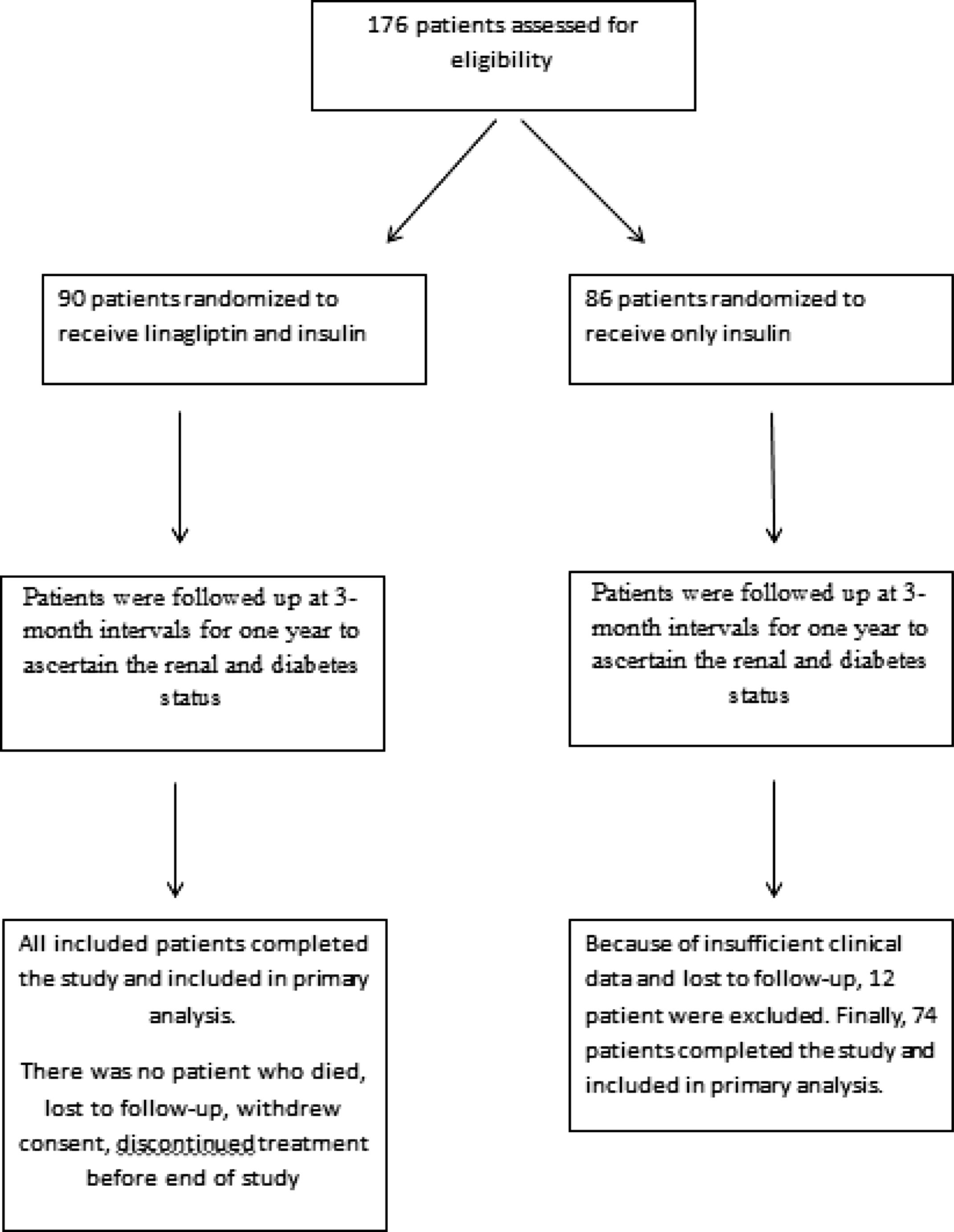
Study participants were randomized into 2 groups by researchers. While patients were randomized, attention was paid to other conditions that might affect renal progression, such as angiotensin converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB) and diuretics usage, be similar among groups. In the first group, linagliptin 5mg was added in addition to the background insulin therapy. In the second group, patients continued their insulin therapy and only insulin dose titration was performed. Patients in the second group did not use any oral antidiabetic medication. Patients were followed up at 3-month intervals for one year to ascertain the renal and diabetes status. Insulin doses were adjusted according to daily glucose measurements. Patients insulin regimen consisted of basal insulin (detemir or U100 glarjin), basal-bolus insulin or mix insulin.
Measurement of renal parameterseGFR was assessed using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, which was shown to perform better than the MDRD (Modification of Diet in Renal Disease) equation with less bias and improved precision.18 Stages of CKD were categorized based on the classification system established by the National Kidney Foundation's Kidney Disease Outcomes (NFK). Renal parameters (blood urea nitrogen, uric acid and creatinine) of patients were measured 5 times during the study period; at enrollment, third, sixth, ninth and twelfth month after enrollment.
Proteinuria was determined by urinary protein excretion (UPCR) from a spot urine sample at baseline and after 3, 6, 9 and 12 months of treatment. UPCR was assessed by the protein-to-creatinine ratio.
Statistical analysisData are expressed as means±SD, medians (minimum–maximum), or numbers (percentages). The normality and the homogeneity of the data were examined by Shapiro–Wilk test and Levene test, respectively. Comparisons between groups for continuous variables were performed using the Student t test (normal distribution) or the Mann–Whitney U test (nonnormal distribution). The Fisher test or χ2 test was used for all categorical data. A paired sample t test was conducted to compare the 2 renal and diabetes status measurements of patients at baseline and 1 year later, respectively. For all calculations, the Statistical Package for the Social Sciences (SPSS, version 15.0; SPSS, Chicago, IL, USA) was used. p<0.05 was considered statistically significant.
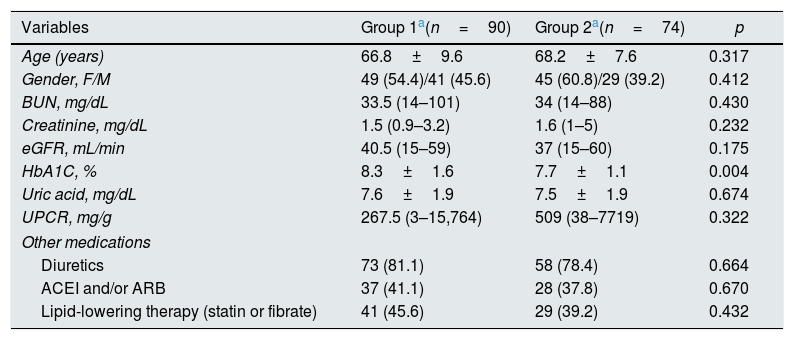
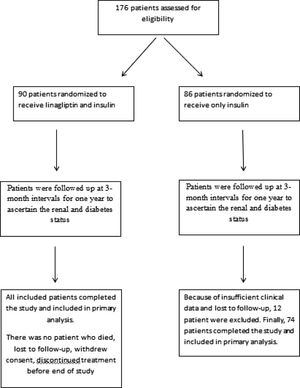
ResultsThe study population consisted of 164 patients with a mean age of 67.5±8.8 years. Patients were divided into two groups after enrollment (Fig. 1). Because of insufficient clinical data and lost to follow-up, 12 patient were excluded from group 2. In group 1, patients (n=90) received linagliptin plus insulin and in group 2, patients (n=74) received only insulin for treatment of diabetes mellitus. Baseline characteristics of the patients according to the groups were shown in Table 1. The patients in the 2 groups were similar with regard to gender, age, eGFR, renal and other laboratory parameters, and other medications; however, group 1 patients had higher HbA1C levels (p=0.004). No patient died during study period. No patients progressed to end-stage renal disease needing chronic dialysis in both groups. None of the patients experienced pancreatitis, hypersensitivity reaction or adverse events leading to study or drug discontinuation.
Basal characteristics between groups.
| Variables | Group 1a(n=90) | Group 2a(n=74) | p |
|---|---|---|---|
| Age (years) | 66.8±9.6 | 68.2±7.6 | 0.317 |
| Gender, F/M | 49 (54.4)/41 (45.6) | 45 (60.8)/29 (39.2) | 0.412 |
| BUN, mg/dL | 33.5 (14–101) | 34 (14–88) | 0.430 |
| Creatinine, mg/dL | 1.5 (0.9–3.2) | 1.6 (1–5) | 0.232 |
| eGFR, mL/min | 40.5 (15–59) | 37 (15–60) | 0.175 |
| HbA1C, % | 8.3±1.6 | 7.7±1.1 | 0.004 |
| Uric acid, mg/dL | 7.6±1.9 | 7.5±1.9 | 0.674 |
| UPCR, mg/g | 267.5 (3–15,764) | 509 (38–7719) | 0.322 |
| Other medications | |||
| Diuretics | 73 (81.1) | 58 (78.4) | 0.664 |
| ACEI and/or ARB | 37 (41.1) | 28 (37.8) | 0.670 |
| Lipid-lowering therapy (statin or fibrate) | 41 (45.6) | 29 (39.2) | 0.432 |
Group 1 patients received linagliptin plus insulin, group 2 patients received only insulin. Data are expressed as the mean±SD, median (minumum–maximum) or noun (percentage), eGFR: estimated glomerular filtration rate, UPCR: urinary protein excretion, F: female, M: male, BUN: blood urea nitrogen, ACEI: angiotensin converting enzyme inhibitors, ARB: angiotensin II receptor blockers.
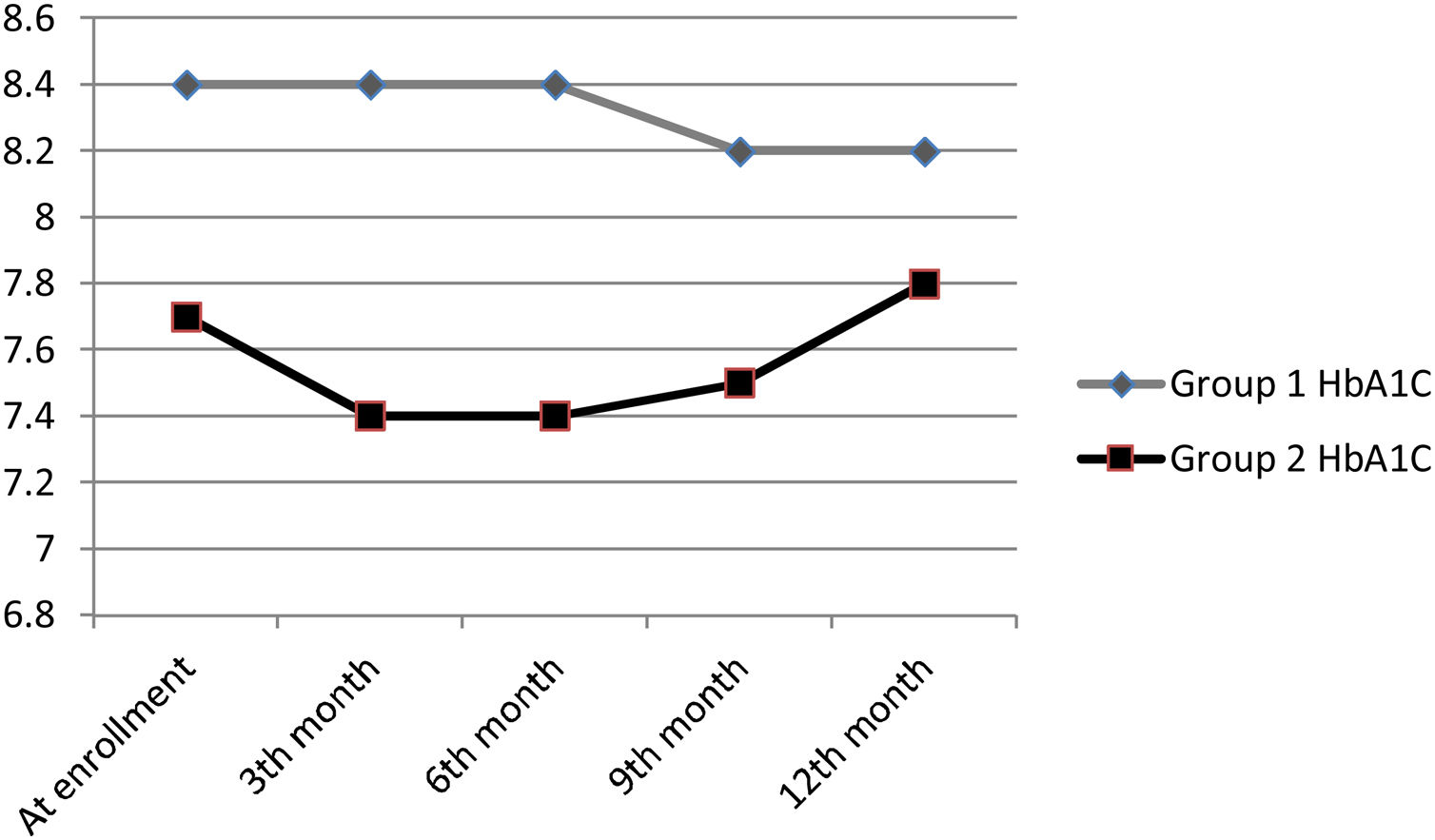
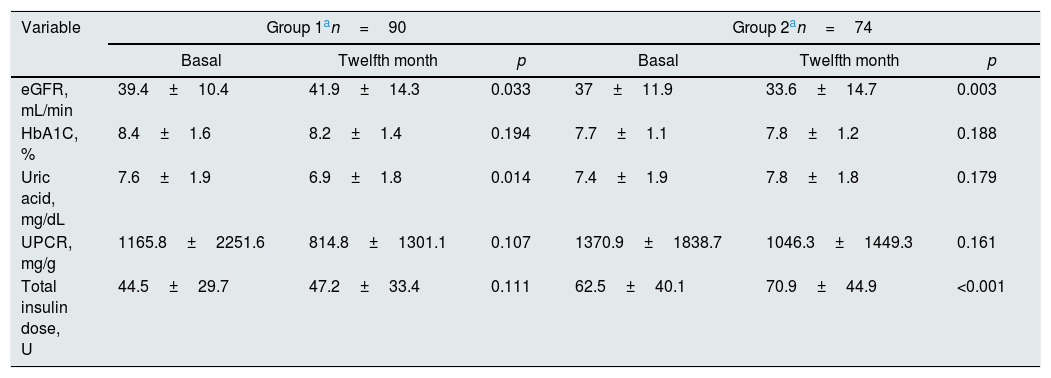
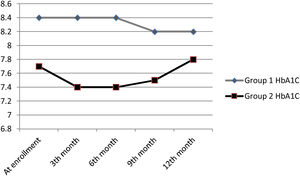
At the end of 12 months follow-up, there was no significant HbA1C change in patients in groups 1 and 2 (Table 2) and HbA1C changes of patients during follow-up were shown in Fig. 2. eGFR significantly increased in group 1 patients (p=0.033), but decreased in group 2 patients (p=0.003). Total insulin dose increased significantly in group 2 (p<0.001), but no significant change was observed in group 1 (p=0.111). While uric acid levels significantly decreased in group 1 (p=0.014), no significant change was observed in group 2 (p=0.179). Proteinuria levels decreased in both groups, but there was no significant change.
Comparison of renal and diabetic parameters between basal and twelfth month measurements.
| Variable | Group 1an=90 | Group 2an=74 | ||||
|---|---|---|---|---|---|---|
| Basal | Twelfth month | p | Basal | Twelfth month | p | |
| eGFR, mL/min | 39.4±10.4 | 41.9±14.3 | 0.033 | 37±11.9 | 33.6±14.7 | 0.003 |
| HbA1C, % | 8.4±1.6 | 8.2±1.4 | 0.194 | 7.7±1.1 | 7.8±1.2 | 0.188 |
| Uric acid, mg/dL | 7.6±1.9 | 6.9±1.8 | 0.014 | 7.4±1.9 | 7.8±1.8 | 0.179 |
| UPCR, mg/g | 1165.8±2251.6 | 814.8±1301.1 | 0.107 | 1370.9±1838.7 | 1046.3±1449.3 | 0.161 |
| Total insulin dose, U | 44.5±29.7 | 47.2±33.4 | 0.111 | 62.5±40.1 | 70.9±44.9 | <0.001 |
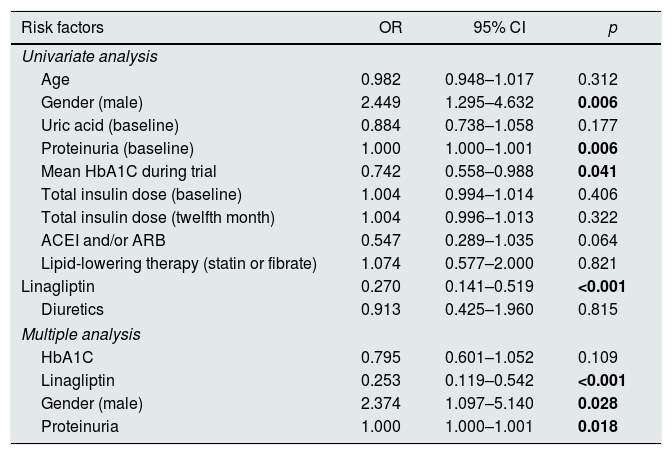
Logistic regression analysis was used to determine the relative risk of progression of renal disease. Only the variables with a statistically significant association in the simple logistic regression model were included in the multiple logistic regression model. Higher proteinuria, male gender emerged as the significant risk factors associated with renal progression in the multiple logistic regression analysis (OR=1.000, 95% CI: 1.000–1.001, p=0.018 and OR=1.755, 95% CI: 1.097–5.140, p=0.028; respectively) (Table 3). Moreover, the use of linagliptin was found to significantly reduce the risk of renal progression in multipl analysis (OR=0.253, 95% CI: 0.119–0.542, p<0.001).
Results of univariate and multiple logistic regression analysis for risk factors for renal progression.
| Risk factors | OR | 95% CI | p |
|---|---|---|---|
| Univariate analysis | |||
| Age | 0.982 | 0.948–1.017 | 0.312 |
| Gender (male) | 2.449 | 1.295–4.632 | 0.006 |
| Uric acid (baseline) | 0.884 | 0.738–1.058 | 0.177 |
| Proteinuria (baseline) | 1.000 | 1.000–1.001 | 0.006 |
| Mean HbA1C during trial | 0.742 | 0.558–0.988 | 0.041 |
| Total insulin dose (baseline) | 1.004 | 0.994–1.014 | 0.406 |
| Total insulin dose (twelfth month) | 1.004 | 0.996–1.013 | 0.322 |
| ACEI and/or ARB | 0.547 | 0.289–1.035 | 0.064 |
| Lipid-lowering therapy (statin or fibrate) | 1.074 | 0.577–2.000 | 0.821 |
| Linagliptin | 0.270 | 0.141–0.519 | <0.001 |
| Diuretics | 0.913 | 0.425–1.960 | 0.815 |
| Multiple analysis | |||
| HbA1C | 0.795 | 0.601–1.052 | 0.109 |
| Linagliptin | 0.253 | 0.119–0.542 | <0.001 |
| Gender (male) | 2.374 | 1.097–5.140 | 0.028 |
| Proteinuria | 1.000 | 1.000–1.001 | 0.018 |
ACEI: angiotensin converting enzyme inhibitors, ARB: angiotensin II receptor blockers, OR: odds ratio, CI: confidence interval.
Development of CKD is one of the major sequelae of type 2 DM.19 Because intensive glucose control per se has not been clearly associated with improvement in renal progression and insulin resistance contributes to the progression of renal disease,20 it is necessary to develop new strategies to improve renal progression. As a new hope in the treatment of diabetic nephropathy, we investigated the effects of linagliptin on renal progression. Patients with advanced CKD have largely been excluded from previous trials of glucose-lowering drugs, resulting in limited available information about use of these drugs in CKD population. This was not the case for the present trial, in which all patients had prevalent CKD. Present study demonstrated that administration of linagliptin markedly slow down renal progression and even improve renal disease in insulin dependent diabetes mellitus patients with CKD. Significantly less insulin was needed in patients using linagliptin. But, linagliptin was not found to be significant in terms of reducing albuminuria. Although patients receiving linagliptin had poorer glucose control at enrollment, a better renal improvement was achieved in patients receiving linagliptin at the end of one year follow up. HbA1c did not significantly change in both groups. The results of present study will provide further guidance on the role of anthyperglycemic therapies in patients with type 2 DM and their impact on the development and progression of diabetic nephropathy.
The risk of renal progression in CKD is determined by many factors. Among these, hyperglycemia and uncontrolled hypertension represent the 2 most frequently studied classic risk factors. On the other hand, strict glycemic control was not associated with any significant changes in renal parameters, such as GFR or urinary albumin excretion, or risk for commencing dialysis in advanced CKD.21–23 These findings suggest that intensive glycemic control may not suffice to prevent renal progression in CKD patients. Similarly, our analysis did not support a direct relationship between renal progression and glucose control. Some other factors could have a stronger impact on renal progression in advanced CKD. Until recently, renal-protective effects of renin-angiotensin system blockers (RASB) were thought to represent a major therapeutic strategy for the management of patients with renal diseases.24 Despite the use of RASB, renal disease continues to progress, and many patients remain proteinuric under treatment. These observations have raised the necessity of an additional strategy in treatment of diabetic patients with CKD. In present study, antihypertensive therapies at enrollment were well balanced between the two treatment groups and significant improvement in renal progression was only detemined in patients using linagliptin at the end of 12 months follow-up. Our results suggest that linagliptin may provide additional benefit in addition to RASB in renal protection.
Compared to other DPP-4 inhibitors, linagliptin is extensively protein bound10 and is mainly eliminated by a biliary route.25 Therefore does not require dose adjustment in patients with CKD.26,27 Although the pharmacokinetics and pharmacodynamics of linagliptin suggests that it will be an ideal agent for patients with CKD, the drug has not adequately been studied in this population.
The mechanism by which linagliptin, which was unable to completely normalize the glucose level, is able to positively modulate kidney function is unknown. Compared to other tissues, the kidneys express the highest level of DPP-4 and it is likely that the presence of DPP-4 in the glomerular endothelium and proximal renal tubules contributes importantly to sodium retention, tubular injury and glomerular injury. Renoprotective effect of linagliptin was probably the result of the inhibition of DPP-4 activity and the enhancement of active glucagon-like peptide-1 (GLP-1) level, which activated GLP-1 receptors, resulting in antioxidative and antiapoptotic effects. The GLP-1 receptor agonist exendin-4 exerts renoprotective effects through its anti-inflammatory action via GLP-1 receptor without glucose control.11 In the Alter et al.’s study, similar effects were achieved by inhibition of DPP-4, which resulted in highly increased plasma GLP-1 levels. In different studies, the renoprotective effect of linagliptin has been associated with various markers, such as osteopontin, cyclophilin A, stromal cell-derived factor-1.6,12,28 Several other experimental studies showed beneficial effects of sitagliptin and vildagliptin on albuminuria and renal function in models of diabetic nephropathy.4,8,11 These preclinical findings raise the possibility of a renal class effect of DPP-4 inhibitors, independent of a glucose-lowering effect. But, it is not possible to extrapolate the results from animal studies into human clinical conditions due to some discrepancies. Prospective, randomized, controlled clinical trials were needed to assess the renal effects of DPP-4 inhibitors in patients with type 2 diabetes. Our study provides very important information in this regard.
Linagliptin significantly reduced albuminuria in Groop et al.’s study.14 Although they found no clinically meaningful change in eGFR during 24 weeks of treatment, our study showed that eGFR improved in patients receiving linagliptin. MARLINA Trial demonstrated the efficacy of linagliptin in improving glycemia in patients with type 2 DM and early diabetic kidney disease, although significant effects on albuminuria were not demonstrated.29 Similarly, our study did not show any effect of linagliptin on proteinuria. These results were unexpected compared to findings in Groop et al.’s study. This difference could have arisen from the limitations of the pooled analysis or differences in the populations.
The number of clinical studies evaluating the renal effects of DPP-4 inhibitors is quite few and the results of these studies were scarce. A randomized study compared sitagliptin with the sulfonylurea in patients with type 2 DM and moderate to severe renal impairment showed that sitagliptin was associated with an increase in UPCR from baseline.30 Ryuge et al.31 found that liraglutide, GLP-1 analog, was not associated with any changes in renal function in patients with diabetic nephropathy. Cooper et al.32 concluded a pooled analysis in patients with type 2 DM and showed that linagliptin was associated with a significant reduction in clinically relevant kidney disease end points (albuminuria, reduction in kidney function). Kim et al demonstrated that DPP-4-inhibitor treatment could ameliorate diabetic nephropathy, by reducing urine albumin excretion and mitigating the reduction of eGFR in T2DM patients.33 But the potential of this drug to improve kidney disease was not clearly established in these studies. Preclinical evidence suggests that the potential renoprotective effects of linagliptin may mostly result from chronic changes in renal physiology rather than acute changes in renal hemodynamics.29,34 Thus, the duration of some studies might not have been sufficient to demonstrate clinically relevant effects on renal function. It is not known whether long-term administration (≥2 years) is required before renal benefits become apparent, as cardiovascular outcomes studies of antihyperglycemic agents. However, our study had a 1-year follow-up and renal functions improved with linagliptin.
CARMELINA trial, a randomized noninferiority trial, evaluated the effects of linagliptin on renal outcomes in patients with type 2 DM and generally more advanced CKD than subjects enrolled in MARLINA trial.35 Unlike our study, CARMELINA trial showed that there was no significant benefit of linagliptin compared with placebo for the incidence of the secondary kidney composite outcome (first occurrence of adjudicated death due to renal failure, end stage renal disease, or sustained 40% or higher decrease in eGFR from baseline). But, present study has important differences from CARMELINA trial. In CARMELINA trial, 38% of study participants did not have low eGFR. But, all patients in our study had prevalent CKD. Another difference was that all of our patients were using insulin. But in the CARMELINA trial, approximately 57% of patients were using insulin. Therefore, our study included a more homogeneous group of CKD patients than the CARMELINA trial. As in our study, notably fewer patients in the linagliptin group initiated or increased doses of preexisting insulin therapy in CARMELINA trial.
Only male gender and proteinuria emerged as significant risk factors for renal progression in our multiple regression analysis at the end of 1-year follow up. Similar to our study, a number of studies suggested that renal disease progression is faster in men and proteinuric patients.36 No significant relationship was found between total insulin dose and renal progression, but the use of linagliptin was found to significantly reduce the risk of renal progression. This result suggests that the improvement in renal function in linagliptin group is not due to the decrease in insulin dose but the use of linagliptin. Possible interactions between renal progression and linagliptin should be investigated in randomized controlled studies.
The rate of progression of CKD shows considerable inter-individual variability and is affected by several factors, such as classic factors (eg, age, gender, ethnicity, family history of CKD, diabetes mellitus, metabolic syndrome, proteinuria and hypertension) or other factors (asymmetric dimethylarginine, fibroblast growth factor 23, calcium–phosphate metabolism, and adiponectin).37,38 Therefore, the major limitation of the present study is that not all factors related to renal progression have been studied. Therefore, this analysis cannot provide conclusive evidence for improved long-term renal outcomes with linagliptin. Another limitation is that UPCR assessments were based on a single urine specimen; this may have reduced the precision of the results because urinary protein excretion shows considerable intraindividual variability.
In conclusion, we have shown that linagliptin in DM patients with CKD was able to improve renal progression without significant effect on proteinuria and glucose control. Despite the availability of many modern therapies for glycemic control, many diabetic patients still progressed to severe renal damage. With regard to treating diabetic nephropathy, blockade of the DPP-4 system may offer a new therapeutic approach.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNo conflicts of interest are declared by any of the authors.