Renal failure is one of the main causes of death in patients with Fabry disease (FD). Due to the low prevalence of FD, delayed diagnosis and misdiagnosis, often the correct diagnosis is made when organ damage is already present. Early recognition of the disease would allow the prevention of severe complications and the premature death of patients with FD.
ObjectiveWe present here the PrEFiNE project, which includes a wide spectrum of activities with the aim of improve knowledge and diagnosis of FD.
The project is sponsored by Shire Iberia (http://shireiberica.com/).
MethodsFrom January 2016 to the end of 2017 several activities will be carried out, starting with a survey to evaluate current FD knowledge among nephrologists; in addition some studies to assess prevalence of this disease will be performed. One study will include patients receiving dialysis, another study will cover kidney transplant patients, and a pilot study in chronic kidney disease in stage 3–5 predialysis. Also planned is a pharmacoeconomic study to focus on burden of FD. At the same time medical education activities will be conducted both on line and on site. Plan for dissemination will include medical publications and diffusion to media. PrEFiNE Project will finish with the publication of a compilation book on FD in Nephrology including all planned activities and proposing recommendations based on results and detected unmet needs.
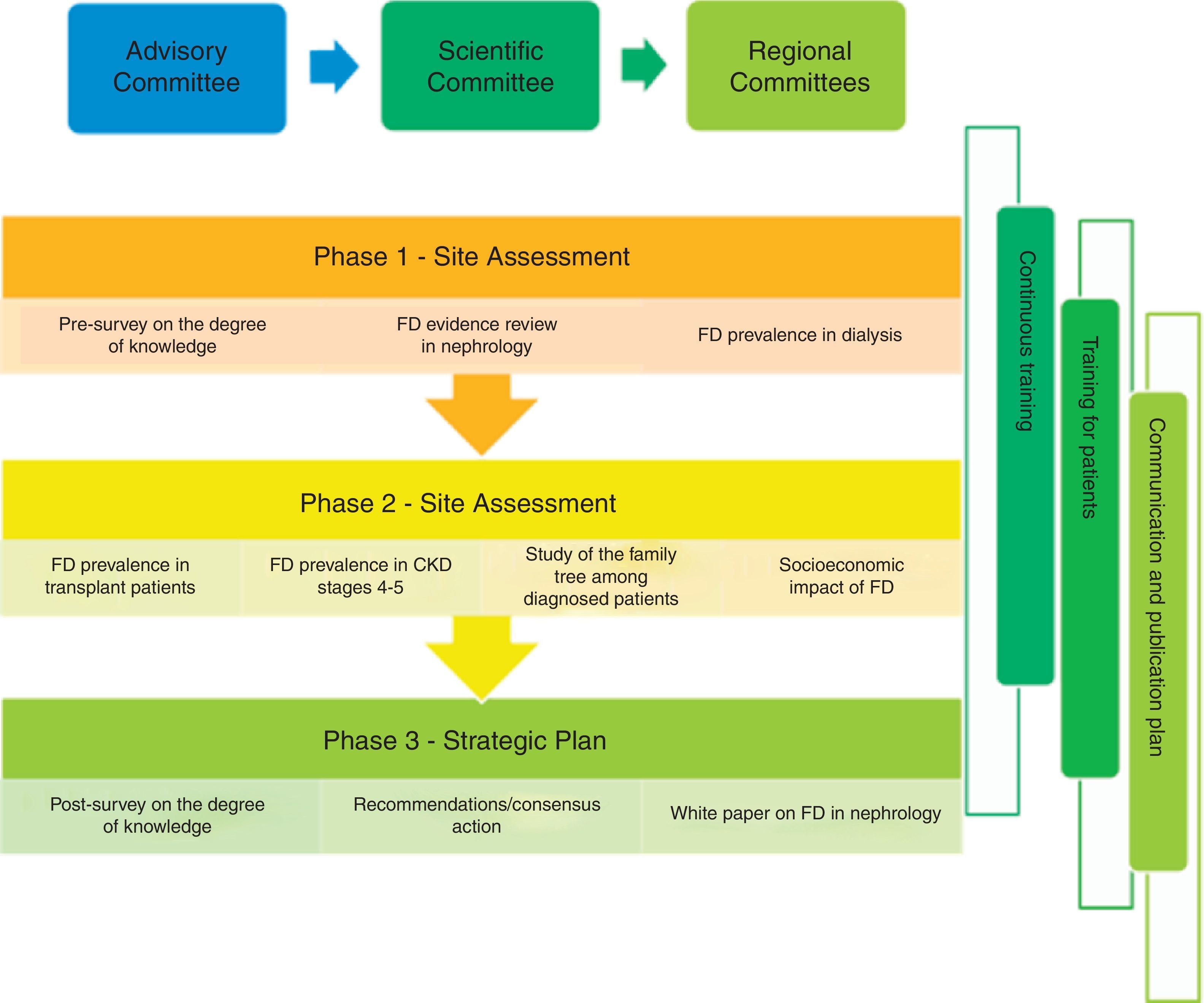
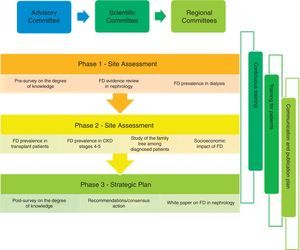
PrEfiNE Plan will be coordinated by severa scientific committees, one at national level and 10 other regionals committees, that will be responsible to ensure the maximum scientific quality of proposed activities. An advisory board will supervise the project.
DiscussionPrEfiNE project will evaluate an action plan focused on improving FD knowledge to make necessary recommendations for an early recognition of the disease. In addition will generate a plan to improve previously undetected needs.
La enfermedad renal es una de las principales causas de muerte entre los pacientes con enfermedad de Fabry (EF). Al tratarse de una enfermedad de baja prevalencia, se realizan con frecuencia diagnósticos erróneos y retrasados, que a menudo se dan cuando ya se ha producido daño orgánico. El reconocimiento temprano de la enfermedad permitiría evitar las complicaciones graves y la muerte prematura en estos pacientes.
ObjetivoPresentamos en este artículo un resumen del plan PrEFiNE, que incluye un abanico amplio de actividades con el objetivo de mejorar el conocimiento y reconocimiento de la EF entre los nefrólogos.
Este proyecto está patrocinado por Shire Ibérica (http://shireiberica.com/).
MétodosDesde enero de 2016 y hasta finales del 2017 se iniciarán distintas actividades, comenzando por una evaluación del grado de conocimiento que existe actualmente sobre la EF. Se incluyen 3 estudios de prevalencia de la EF, que abarcan el espectro de los pacientes con enfermedad renal crónica (pacientes en diálisis, pacientes trasplantados renales y un estudio piloto en pacientes con enfermedad renal crónica en estadio 3–5 prediálisis) y un estudio farmacoeconómico, centrado en el impacto de la carga de la enfermedad. Paralelamente, se realizarán actividades formativas tanto presenciales como on line, y un amplio plan de comunicación mediante publicaciones y difusión a medios. El proyecto culminará con la publicación de un libro blanco de la EF en Nefrología, que recoja el resultado de todas las actividades y que proponga recomendaciones en respuesta a los resultados y a las necesidades detectadas.
El plan PrEFiNE estará coordinado por distintos comités científicos, uno nacional y 10 regionales que garantizarán el desarrollo de las acciones con el máximo rigor científico y será supervisado por un comité asesor.
DiscusiónEl plan PrEFiNE nos permitirá evaluar la utilidad de un proyecto dirigido a mejorar el conocimiento de una enfermedad minoritaria como la EF a nivel nacional, y a partir del cual se podrán establecer las recomendaciones necesarias para mejorar su reconocimiento, además de planes enfocados a mejorar las necesidades no cubiertas detectadas durante su desarrollo.
Fabry's disease (FD) is a lysosomal disease that is characterised by a deficiency in α-galactosidase A, an enzyme that causes glycosphingolipids, mainly globotriaosylceramide (Gb3), to be deposited in the vascular endothelium and other tissues. It is a progressive disease that causes manifestations in the kidneys, heart, nervous system, skin, eyes and other systems.1,2
It is considered to be a rare disease, as it affects 1 in 40,000–238,000 males.2–4 However, studies conducted using neonatal screening have observed a greater incidence, of 1/3100 to 1/4100 live births for late-onset forms and 1/37,000 for classic phenotypes.5,6 Obviously, its frequency is greater in high-risk populations such as the population affected by chronic kidney disease (CKD) that requires dialysis or the population suffering from by left ventricular hypertrophy or stroke.7 Life expectancy is normally shortened by 20 years in men8 and 15 years in women.9 One of the main causes of death in patients with FD is renal failure and it occurs very early, in the fourth or fifth decade of life, particularly in males.10,11
One analysis of patients with FD determined that more than 25% had been diagnosed incorrectly initially12 and that their diagnosis took an average of 12 years to confirm.13 Diagnosis tends to be delayed by at least 3 years, but it often takes 20 years after the onset of signs and symptoms. Some patients have even experienced delays of more than 50 years.12 Patients with FD and already impaired renal function have a more unfavourable prognosis than those without it.14
In clinical practice there is no unanimous agreement for including FD as part of the differential diagnosis of patients with CKD in any stage. This makes it difficult to diagnose the disease early, identify at-risk family relatives and prevent other secondary and potentially deadly complications of the disease.
As generally occurs with rare diseases, part of the problem may lie in limited awareness of the disease such that a diagnosis of FD may not be considered as part of the diagnostic algorithm for patients with CKD.
The PrEFiNE plan was created with the aim of improving knowledge and recognition of FD among all nephrologists, which would allow an early diagnosis of the disease and an early approach to patients who have it. The aims of this plan are many and range from evaluating current knowledge of FD and establishing its prevalence in patients with CKD (dialysis, transplant and stage 3–5 CKD predialysis); evaluating the impact of FD from a healthcare-related, sociological and economic point of view; improving knowledge of FD through specific training in the disease; detecting potential unmet needs in the approach to FD; and, finally, developing a prioritised action plan to improve the needs detected and reduce the repercussions of the disease.
Therefore, this multi-centre project, encompassing all of Spain, is being proposed through a broad training and information campaign, with continuing education and enhancement of different research studies.
The intention of this article is to spread information on the design of this project and some of its activities to offer it as a platform that facilitates and stimulates research on and knowledge of FD among Spanish nephrologists.
MethodologyGeneral designThe project will have an advisory committee headed by the chairperson of the Spanish Society of Nephrology (SEN) who will be in charge of driving, promoting and reviewing the project; advising on the proposed aims; and involving all participants effectively.
A scientific committee will validate the general approach of the project and the actions and studies to be carried out; advise on the mode in which each action will be performed, thereby ensuring maximum scientific rigour; select and produce required training materials (in person and online), review the results obtained; and propose and reach an agreement on future lines of research.
Regional committees will coordinate and execute the training activities required to improve knowledge of FD in their region; advise on the mode in which each action will be performed in the regions, thereby ensuring maximum scientific rigour; review the results obtained in the area; and propose and reach an agreement on future lines of research.
The following actions are proposed (Fig. 1):
- •
Three multi-centre prevalence studies, which will be carried out in accordance with the ethics requirements of the declarations of Helsinki for research with human beings, the good epidemiological practice guidelines of the International Conference on Harmonisation and the existing standards in Spain, and which will be approved by the relevant ethics committees:
- ∘
In patients in dialysis, both haemodialysis and peritoneal dialysis, with non-restrictive inclusion criteria.
- ∘
In renal transplant patients.
- ∘
In patients with stages 3–5 CKD predialysis; in this case the study is proposed as a pilot.
- ∘
- •
The protocols for the studies will be available on the website enabled for the project. Access by and participation of any SEN member will require prior approval of the project by the scientific committee.
- •
Study of the family tree among patients diagnosed with FD.
- •
The degree of knowledge of FD among nephrologists will be quantified before and after the development of the plan through a brief survey; in this way the training needs of all of them with respect to the disease will be detected.
- •
University courses focused on FD and clinical genetics, and regional on-site training sessions.
- •
An evidence-based review with analysis of all available information on FD in the field of Nephrology.
- •
A pharmacoeconomic study of the economic burden of FD for society, the individual and the healthcare system.
- •
Finally, the results obtained from the projects and activities performed will be collected and a white paper on FD will be prepared that may serve as a basis for preparing recommendations for early diagnosis of the disease in Nephrology.
In the course of the project the different committees will analyse the progress of the activities and studies, and propose actions to improve those activities that require it. At least 3 annual meetings are planned for each committee.
Methodology of the epidemiological studiesIn the prevalence studies and the analysis of the relatives of patients diagnosed with FD, after obtaining informed consent from all participants, a specific electronic case report form (e-CRF) will be filled in for each study; all will be available for restricted use by the investigators on the project website (www.prefine.es). The e-CRF will include sociodemographic variables, clinical disease events, any complications or impairment of organs or systems that the patient may have and the dates on which the patient started to have symptoms suggestive of FD. As these are epidemiological projects of prevalence in an at-risk population, the aim is to screen as many patients as possible. It is estimated that up to 10,000 dialysis patients and 3000 transplant patients will be reached. The number of CKD patients in the study has not been estimated as this is a pilot study. Patient selection will be performed by means of consecutive sampling of patients who visit Nephrology outpatient clinics or are cared for at dialysis centres. Patients of both sexes who meet the inclusion criteria for each study will be accepted, without any age limit. The dialysis study protocol has been approved by the Hospital Clínic de Barcelona Ethics Committee, and the transplant study protocol has been approved by Hospital de Bellvitge in L’Hospitalet de Llobregat (Barcelona). The CKD protocol is pending evaluation by the Basque Country IEC.
All patients selected to participate will undergo a dried blood spot analysis; male patients will undergo determination of their α-galactosidase A enzyme activity, and when this is below normal limits, they will undergo a genetic study of the GLA gene; women will undergo a genetic study regardless of their enzyme activity result. All patients will undergo determination of their lyso-Gb3 value.
A blood sample will be drawn from consenting patients and stored in the RedinRen biobank. The sample will allow future research to be conducted in these populations.
Patients with a positive result for FD will be evaluated according to routine clinical practice at each site or will be referred to specialised sites with experience in management of the disease, at the discretion of each investigator.
The SPSS statistical programme will be used for data analysis. Descriptive statistical analysis of the variables will be performed. A statistical significance level of 0.05 will be used. Preliminary statistical techniques will be used when performing hypothesis-verifying tests to ensure that statistical assumptions are met. In the event that the established assumptions are not met, equivalence tests without these limitations, such as non-parametric tests, will be used.
DiscussionThe PrEFiNE project is the first comprehensive plan to improve knowledge of a rare disease in the field of a medical speciality. It may serve as a pilot project to improve knowledge of rare diseases in target specialities.
Our hypothesis is that the range of activities aimed at all nephrologists will allow knowledge and recognition of FD to be improved and recommendations that facilitate management of these patients to be established.
Therefore, it is hoped that this project will lead to a shift in the current paradigm in diagnosis of the disease such that time to diagnosis decreases and the long-term complications that these patients have may be reduced.
The results obtained will be very useful as they will allow comparison between the results for the different stages of CKD and subsequently comparison between these results and results obtained in future studies.
However, to achieve the proposed objectives, this project requires the participation of the majority of Nephrology Departments, which will be involved in both the training activities and the proposed lines of research. The data generated during the project will follow the data use standards of the SEN and will be transferred to the SEN once all proposed objectives have been met.
Authorship standards will be established by the scientific committee of the project.
We would like this study to be considered to be a common enterprise of the nephrologists of the SEN. It is also our intention that this article which lays out the activities to be performed stimulates collaboration to achieve the desired objective.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: del Pino MD, Ortiz A, Torra R, Hernandez D. Plan PrEFiNE: Plan estratégico para la enfermedad de Fabry en Nefrología. Nefrología. 2016;36:376–380.