One in 10 patients with hyperuricemia may develop gout over time, with urate deposition sometimes asymptomatic. Recent reviews and guidelines support ultrasound (US) to assess asymptomatic hyperuricemic (AH) patients to detect gout lesions, showing double contour (DC) and tophus the highest specificities and positive predictive values. Hyperuricemia and gout are common in chronic kidney disease (CKD), especially with glomerular filtration rate (GFR) <60, and both are associated with worse prognosis, although treatment of AH in CKD is not yet recommended in all guidelines. US gout lesions have been found more frequently in AH (up to 35%) than in normouricemic (NU) patients, but evidence is scarce in CKD.
ObjectivesTo assess the prevalence of urate deposit in stages 3–5 CKD detected by US, and to investigate if there are differences between AH and NU patients.
MethodsMulticenter cross-sectional study, recruiting patients aged ≥18 years with AH and stages 3–5 CKD in 4 hospitals. A comparator group of NU patients with stage 3–5 CKD was included. Exclusion criteria: previous diagnosis of gout, tophi. Hyperuricemia was defined as serum uric acid (sUA) >7 mg/dl, documented at least twice during the last 12 months. A standardized US exam of the knees and bilateral first metatarsophalangeal joints was performed to assess patients for DC/tophus as defined by OMERACT. Demographic, clinical and laboratory data were recorded. A descriptive analysis was performed using SPSS. Pre-clinical gout (PCG: DC and/or tophus) was considered as outcome variable. Chi-square and Fisher's exact test were used for qualitative variables, and Mann–Whitney U test for quantitative variables; significant threshold p < 0.05.
ResultsFifty-three patients with stages 3–5 CKD (59.6% stage 3, 19.1% stage 4, 21.3% stage 5) were recruited, 38 AH (71.7%) and 15 NU. A higher prevalence of US findings was observed in HU patients compared to NU patients (DC 23.7% vs 13.3%, tophus 31.6% vs 26.7%, PCG 39.5% vs 33.3%), although the differences were not statistically significant. NU patients had CKD of longer duration than HU patients [11 (7.2–13.5) vs 6 (2–9.2) years; p = 0.02], with no differences in sex, age, comorbidities, or urate-lowering therapy (ULT) (66.7% vs 44.7%; p = 0.05) and other treatments. Seventy percent of NU patients with TRU had AH before starting treatment. In patients with tophi, we observed a trend towards shorter duration of CKD and shorter duration of treatment with ULT compared to those without tophi [3.5 (2−6.7) vs 7 (3−12) years; p = 0.05] and [22 (12−44) vs 39 (29−73) months; p = 0.08], respectively. This trend was also observed in PCG, but not in DC, first US sign to disappear after initiation of ULT. Ninety percent of patients (100% in non-dialyzed patients) with PCG had a median uricemia ≥5 mg/dl in the past 12 months.
ConclusionWe found a significant prevalence of asymptomatic urate deposition in patients with stage 3–5 CKD, mostly in subjects with median uricemia ≥5 mg/dl in the last 12 months. Early diagnosis of PCG by musculoskeletal US in CKD may allow earlier introduction and optimization of ULT. This will probably contribute to slowing down the progression of this pathology, which makes it essential to promote collaboration between Nephrology and Rheumatology.
Uno de cada 10 pacientes con hiperuricemia desarrolla gota. Estudios recientes apoyan la ecografía para detección de gota en pacientes con hiperuricemia asintomática (HUA), pues el depósito articular de urato suele ser asintomático en etapas iniciales. Doble contorno (DC) y tofo son las lesiones con mejor especificidad y valor predictivo positivo. En enfermedad renal crónica (ERC), hiperuricemia y gota son más frecuentes que en población general, especialmente en pacientes con filtrado glomerular (FGE) <60, asociándose a peor pronóstico renal, aunque el tratamiento de HUA en ERC no aparece recomendado en todos los documentos de consenso. Las lesiones ecográficas de gota aparecen más frecuentemente en pacientes con HUA (hasta 35%) que en normouricémicos, pero se desconoce si esto también ocurre en ERC. Nuestro objetivo es evaluar prevalencia de depósito articular asintomático de urato en ERC estadio 3–5, y analizar si existen diferencias entre hiperuricémicos y normouricémicos.
Pacientes y métodosEstudio transversal multicéntrico con pacientes con HUA y ERC estadio 3–5, incliuyendo grupo comparador de normouricémicos. Se excluyeron pacientes con diagnóstico previo de gota o tofos en exploración. Se definió hiperuricemia como ácido úrico sérico (AUS) >7 mg/dl en ≥2 analíticas en últimos 12 meses. Se realizó ecografía bilateral de rodilla y primera metatarsofalángica. Se registraron datos demográficos, clínicos y de laboratorio. Se realizó análisis descriptivo de la muestra, evaluándose frecuencia de lesiones ecográficas de gota. Se definió la variable de resultado como presencia/ausencia de gota preclínica (GPC: DC y/o tofo), utilizándose pruebas de Chi-cuadrado y Fisher para comparación de variables cualitativas, y U de Mann-Whitney para cuantitativas. Nivel de significación p < 0.05 (SPSS v21).
ResultadosSe reclutaron 53 pacientes con ERC: 38 (71.7%) con HUA y 15 normouricémicos. 37 pacientes eran varones (69.8%), mediana de edad 73 años (64.5−77.5). Se detectaron tofos en 16 pacientes (30.2%), DC en 11 (20.8%) y GPC en 20 (37.7%). Se observó mayor prevalencia de hallazgos en hiperuricémicos que en normouricémicos (DC: 23.7% vs 13.3%; tofo: 31.6% vs 26.7%; GPC: 39.5% vs 33.3%), aunque las diferencias no fueron estadísticamente significativas. Los normouricémicos tenían ERC de mayor duración que los hiperuricémicos [11 (7.2−13.5) vs 6 (2−9.2) años; p = 0.02], sin diferencias en sexo, edad, comorbilidades, reductor de uricemia (TRU) (66.7% vs 44.7%; p = 0.05) u otros fármacos. El 70% de normouricémicos con TRU tenía hiperuricemia previamente a su inicio. En pacientes con tofos observamos tendencia a menor duración de ERC y del tratamiento con TRU frente a aquellos sin tofos [3.5 (2−6.7) vs 7 (3−12) años; p = 0.05] y [22 (12−44) vs 39 (29−73) meses; p = 0.08], respectivamente. Esta tendencia también se observó en GPC, no así en DC. 90% de pacientes (100% en no dializados) con GPC tenían mediana de uricemia ≥5 mg/dl en últimos 12 meses.
ConclusionesEncontramos importante prevalencia de depósito articular asintomático de urato en pacientes con ERC estadio 3–5, predominantemente en aquellos con mediana de uricemia ≥5 mg/dl en últimos 12 meses. El diagnóstico precoz de GPC mediante ecografía en ERC puede ayudar a adelantar y optimizar el tratamiento con TRU. Es imprescindible potenciar la colaboración nefrología-reumatología.
Gout is a disease caused by the deposition of monosodium urate (MSU) crystals in the tissues. It is a prevalent and severe systemic condition, with significant articular, renal and cardiovascular effects.1,2 Hyperuricaemia, i.e., serum uric acid (SUA) levels >7 mg/dl, maintained over time may produce gout. According to different studies, the incidence of gout in patients with hyperuricaemia with no prior symptoms may vary broadly, between 0.5% and 49%, depending on the level of uricaemia and the time elapsed.3–5
In the early years of the disease, the initial symptoms of this disease may be non-specific and subtle, even non-existent. The latest recommendations by the European Alliance of Associations for Rheumatology (EULAR) for the diagnosis of gout place the silent deposition of MSU crystals at an intermediate stage between asymptomatic hyperuricaemia (AHU) and gout.6 However, some authors already consider that asymptomatic deposit of MSU in patients with hyperuricaemia should be contemplated as 'preclinical' gout (PCG).7,8
Hyperuricaemia and gout are common in patients with renal disease. Data from registries and population studies show that one in four patients with gout has chronic kidney disease (CKD) stage ≥3, and that 24% of patients with CKD suffer from gout, with the prevalence rising to 35.6 % in those with an estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2.9–11 The prevalence of hyperuricaemia is also directly related to a decrease in eGFR; it is found in 40–60% of patients with stage 1–3 CKD, and in 70% with stage 4–5.12,13 Hyperuricaemia, and especially gout, worsen the prognosis of patients with CKD and, although there is not universal consensus, urate-lowering therapy (ULT) could contribute to reverse the process, improving both joint and renal damage.1,2,14
Early diagnosis of gout in the preclinical or paucisymptomatic phase is a challenge for rheumatologists, nephrologists and primary care physicians. Imaging tests, such as dual-energy computerised tomography (DECT) and joint ultrasound (widely used as it is harmless, fast and more affordable), have been postulated in recent years as useful tools for the diagnosis of silent deposits of MSU crystals (detected by DECT in 15–24% of patients with AHU, and by ultrasound in 13–42%) and have therefore been included in the latest international recommendations.6,15–21
To the best of our knowledge, to date there have been no previous publications on the detection of PCG in CKD using ultrasound. Therefore, the objectives of this study are to determine the prevalence of silent deposition of MSU crystals in stage 3–5 CKD patients using joint ultrasound, and to investigate whether there are differences in joint ultrasound findings between hyperuricaemic patients and those with normal serum uric acid levels.
Patients and methodsType of study and patient selectionThis is a multicentre cross-sectional study, with approval from the Infanta Leonor-Virgen de la Torre University Hospital Independent Ethics Committee (HUIL-19/00), supported by a SORCOM-MSD grant (Madrid Rheumatology Society of the Community of Madrid and Merck Sharp and Dohme) awarded to Dr Leticia Lojo Oliveira.
Inclusion criteria: patients ≥18 years of age with signed informed consent and diagnosis of stage 3–5 CKD according to the definition of the Kidney Disease Outcomes Quality Initiative (KDOQI): kidney damage (albuminuria; urine albumin-creatinine ratio >30 mg/g) or eGFR <60 ml/min/1.73 m2 for ≥3 months, regardless of cause. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula was used to determine the eGFR. Based on the serum uric acid levels, patients were classified as cases if they presented with hyperuricaemia (SUA > 7 mg/dl) in ≥2 tests in the last 12 months, and as a comparator group those with normal serum uric acid levels.
Exclusion criteria: patients with a previous diagnosis of gout, characteristic symptoms of gout (current or past) according to the patient's medical history, or tophi in the physical examination.
Joint ultrasound studyThe same rheumatologist responsible for collecting the medical history and performing the physical examination at each site carried out the systematic bilateral B-mode ultrasound examination of the knee and first metatarsophalangeal joint (1MTP) with a linear probe >7.5 MHz, following the protocol defined by De Miguel et al.17 There are other published proposals for ultrasound examination in patients with gout, but we opted for this protocol given the easiness and speed of its performance in a routine consultation, and also its good balance between sensitivity and specificity.
The ultrasound examination was considered to be consistent with PCG, the outcome variable of the study, if it showed tophi and/or the double contour (DC) sign, the two most suggestive elementary ultrasound lesions of gout according to Outcome Measures in Rheumatology Clinical Trials (OMERACT).22
A unified evaluation and interpretation of the images was performed by an external central observer, blinded to the patients' clinical data. The degree of concordance between the independent observers and the central observer was subsequently calculated. In cases of a lack of agreement in the interpretation, a second reading was performed by another external observer.
Data collection and storagePatient recruitment ran from January 2020 to April 2022, with the initially planned deadlines having to be changed owing to the COVID pandemic.
An anonymised database was designed in which the following variables were entered: age, sex, body mass index (BMI), family history of gout, history of urolithiasis, smoking, alcohol consumption, CKD characteristics (time since onset, stage, haemodialysis treatment), lab test parameters (baseline and previous 12 months: SUA, serum creatinine, albuminuria, eGFR), tophi or DC on ultrasound examination. Comorbidities such as arterial hypertension (HTN), diabetes mellitus (DM), dyslipidaemia (DL), ischaemic heart disease and cerebrovascular disease, as well as concomitant treatments (urate-lowering therapy, diuretics, nonsteroidal anti-inflammatory drugs, acetylsalicylic acid and lipid-lowering agents) were also collected and included in the database.
The ultrasound images from the four sites were anonymised and stored together in a single file, so that the identity of each independent investigator could not be determined.
Data processing and analysisStatistical analysis: a descriptive analysis of the sample was performed, expressing the qualitative variables as absolute and relative frequencies, and the quantitative variables as means and medians, with their respective measures of dispersion (standard deviation [SD] and interquartile range [IQR]). Differences between qualitative and quantitative variables were analysed using the X2 test, Student's t-test and Mann–Whitney U test, respectively. We considered the main dependent variable to be the finding of ultrasound signs consistent with MSU deposition, understood as a dichotomous qualitative variable (deposition versus non-deposition). To assess the association of this variable with the independent variables, a bivariate analysis was performed. Statistical significance: p < 0.05. We used SPSS (IBM, version 21.0) for all analyses.
To calculate the degree of agreement between independent observers and the central observer, the proportion of agreements observed was used as the concordance index, with values between 0 (total disagreement) and 1 (maximum agreement).
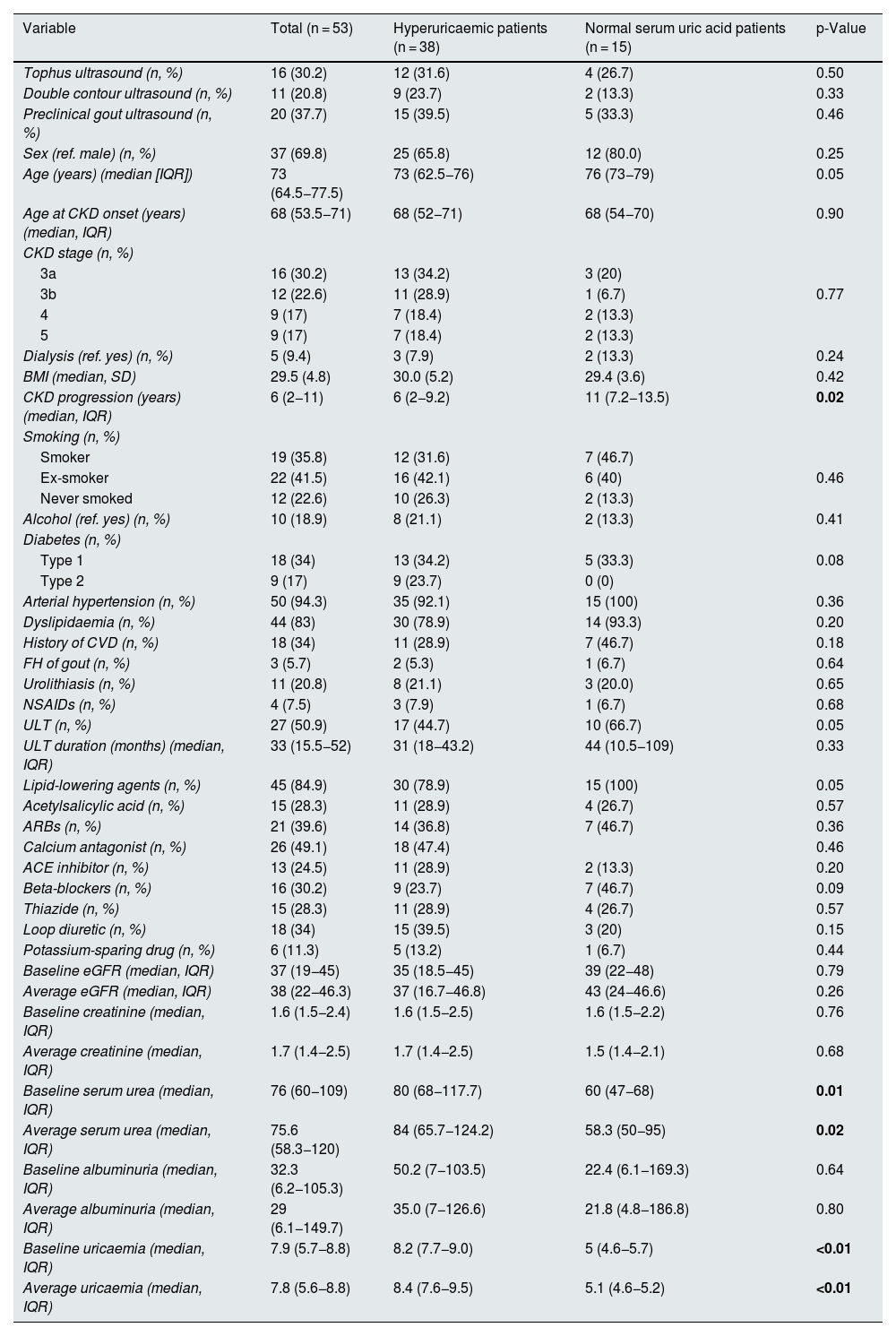
ResultsIn total, 53 patients were recruited (38 with hyperuricaemia and 15 with normal serum uric acid levels), with a median age of 73 (64.5−77.5) years, most with CKD stage 3 (53%). The demographic characteristics of the sample, as well as the duration of CKD, laboratory test parameters, treatments and comorbidities, are listed in Table 1.
Patient characteristics, laboratory test parameters and ultrasound findings.
| Variable | Total (n = 53) | Hyperuricaemic patients (n = 38) | Normal serum uric acid patients (n = 15) | p-Value |
|---|---|---|---|---|
| Tophus ultrasound (n, %) | 16 (30.2) | 12 (31.6) | 4 (26.7) | 0.50 |
| Double contour ultrasound (n, %) | 11 (20.8) | 9 (23.7) | 2 (13.3) | 0.33 |
| Preclinical gout ultrasound (n, %) | 20 (37.7) | 15 (39.5) | 5 (33.3) | 0.46 |
| Sex (ref. male) (n, %) | 37 (69.8) | 25 (65.8) | 12 (80.0) | 0.25 |
| Age (years) (median [IQR]) | 73 (64.5−77.5) | 73 (62.5−76) | 76 (73−79) | 0.05 |
| Age at CKD onset (years) (median, IQR) | 68 (53.5−71) | 68 (52−71) | 68 (54−70) | 0.90 |
| CKD stage (n, %) | ||||
| 3a | 16 (30.2) | 13 (34.2) | 3 (20) | |
| 3b | 12 (22.6) | 11 (28.9) | 1 (6.7) | 0.77 |
| 4 | 9 (17) | 7 (18.4) | 2 (13.3) | |
| 5 | 9 (17) | 7 (18.4) | 2 (13.3) | |
| Dialysis (ref. yes) (n, %) | 5 (9.4) | 3 (7.9) | 2 (13.3) | 0.24 |
| BMI (median, SD) | 29.5 (4.8) | 30.0 (5.2) | 29.4 (3.6) | 0.42 |
| CKD progression (years) (median, IQR) | 6 (2−11) | 6 (2−9.2) | 11 (7.2−13.5) | 0.02 |
| Smoking (n, %) | ||||
| Smoker | 19 (35.8) | 12 (31.6) | 7 (46.7) | |
| Ex-smoker | 22 (41.5) | 16 (42.1) | 6 (40) | 0.46 |
| Never smoked | 12 (22.6) | 10 (26.3) | 2 (13.3) | |
| Alcohol (ref. yes) (n, %) | 10 (18.9) | 8 (21.1) | 2 (13.3) | 0.41 |
| Diabetes (n, %) | ||||
| Type 1 | 18 (34) | 13 (34.2) | 5 (33.3) | 0.08 |
| Type 2 | 9 (17) | 9 (23.7) | 0 (0) | |
| Arterial hypertension (n, %) | 50 (94.3) | 35 (92.1) | 15 (100) | 0.36 |
| Dyslipidaemia (n, %) | 44 (83) | 30 (78.9) | 14 (93.3) | 0.20 |
| History of CVD (n, %) | 18 (34) | 11 (28.9) | 7 (46.7) | 0.18 |
| FH of gout (n, %) | 3 (5.7) | 2 (5.3) | 1 (6.7) | 0.64 |
| Urolithiasis (n, %) | 11 (20.8) | 8 (21.1) | 3 (20.0) | 0.65 |
| NSAIDs (n, %) | 4 (7.5) | 3 (7.9) | 1 (6.7) | 0.68 |
| ULT (n, %) | 27 (50.9) | 17 (44.7) | 10 (66.7) | 0.05 |
| ULT duration (months) (median, IQR) | 33 (15.5−52) | 31 (18−43.2) | 44 (10.5−109) | 0.33 |
| Lipid-lowering agents (n, %) | 45 (84.9) | 30 (78.9) | 15 (100) | 0.05 |
| Acetylsalicylic acid (n, %) | 15 (28.3) | 11 (28.9) | 4 (26.7) | 0.57 |
| ARBs (n, %) | 21 (39.6) | 14 (36.8) | 7 (46.7) | 0.36 |
| Calcium antagonist (n, %) | 26 (49.1) | 18 (47.4) | 0.46 | |
| ACE inhibitor (n, %) | 13 (24.5) | 11 (28.9) | 2 (13.3) | 0.20 |
| Beta-blockers (n, %) | 16 (30.2) | 9 (23.7) | 7 (46.7) | 0.09 |
| Thiazide (n, %) | 15 (28.3) | 11 (28.9) | 4 (26.7) | 0.57 |
| Loop diuretic (n, %) | 18 (34) | 15 (39.5) | 3 (20) | 0.15 |
| Potassium-sparing drug (n, %) | 6 (11.3) | 5 (13.2) | 1 (6.7) | 0.44 |
| Baseline eGFR (median, IQR) | 37 (19−45) | 35 (18.5−45) | 39 (22−48) | 0.79 |
| Average eGFR (median, IQR) | 38 (22−46.3) | 37 (16.7−46.8) | 43 (24−46.6) | 0.26 |
| Baseline creatinine (median, IQR) | 1.6 (1.5−2.4) | 1.6 (1.5−2.5) | 1.6 (1.5−2.2) | 0.76 |
| Average creatinine (median, IQR) | 1.7 (1.4−2.5) | 1.7 (1.4−2.5) | 1.5 (1.4−2.1) | 0.68 |
| Baseline serum urea (median, IQR) | 76 (60−109) | 80 (68−117.7) | 60 (47−68) | 0.01 |
| Average serum urea (median, IQR) | 75.6 (58.3−120) | 84 (65.7−124.2) | 58.3 (50−95) | 0.02 |
| Baseline albuminuria (median, IQR) | 32.3 (6.2−105.3) | 50.2 (7−103.5) | 22.4 (6.1−169.3) | 0.64 |
| Average albuminuria (median, IQR) | 29 (6.1−149.7) | 35.0 (7−126.6) | 21.8 (4.8−186.8) | 0.80 |
| Baseline uricaemia (median, IQR) | 7.9 (5.7−8.8) | 8.2 (7.7−9.0) | 5 (4.6−5.7) | <0.01 |
| Average uricaemia (median, IQR) | 7.8 (5.6−8.8) | 8.4 (7.6−9.5) | 5.1 (4.6−5.2) | <0.01 |
ACE inhibitors: angiotensin-converting enzyme inhibitors; ARBs: angiotensin II receptor blockers; BMI: body mass index; CKD: chronic kidney disease; CVD: cardiovascular disease; eGFR: estimated glomerular filtration; FH: family history; NSAIDs: nonsteroidal anti-inflammatory drugs; ULT: urate-lowering therapy. Statistical significance: p < 0.05 (values in bold).
Among patients with normal serum uric acid levels, 66.7% were on ULT at the time of inclusion versus 44.7% of patients with hyperuricaemia (p = 0.05), with a longer period since onset of CKD (11 [7.2−13.5] vs 6 [2−9.2] years; p = 0.02) and lower serum urea values (60 [47−68] vs 80 [68−117.7]; p = 0.01) in normal serum uric acid patients than hyperuricaemic patients. Similarly, the treatment time with ULT was longer in patients with normal serum uric acid levels (44 [10.5−109] months) than in hyperuricaemic patients (31 [18–42.2] months), although these differences were not statistically significant. There were no significant differences in sex, age, comorbidities or other treatments between patients with normal serum uric acid levels and hyperuricaemic patients (Table 1). In patients with normal serum uric acid levels receiving ULT, a 70% had hyperuricaemia prior to the start of the treatment, half of them with values higher than 8 mg/dl (range 7.2–10.9).
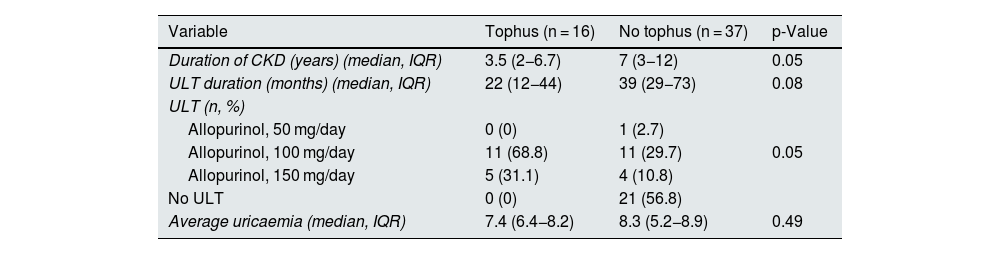
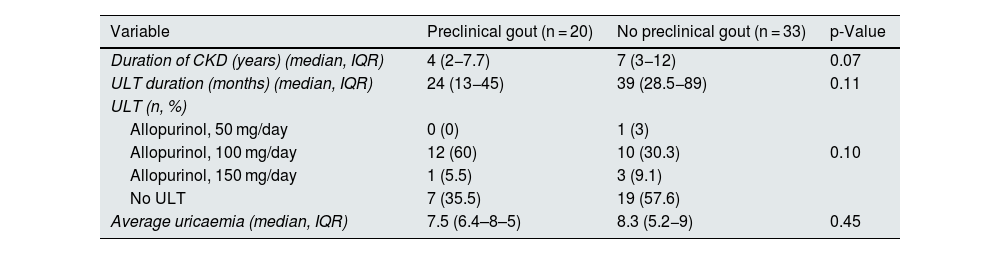
Regarding ultrasound findings, tophi were detected in 16 patients of the total sample (30.2%), DC in 11 patients (20.8%) and PCG in 20 patients (37.7%) (Table 1). Ultrasound findings were more prevalent in hyperuricaemic patients than in normal serum uric acid patients, although these differences were not statistically significant. In patients in whom tophi were detected, there was a tendency towards a shorter duration of CKD (3.5 [2−6.7] vs 7 [3–12] years, p = 0.05]) and shorter duration of treatment with ULT as compared to those without tophi (22 [12−44] vs 39 [29−73] months [p = 0.08]) (Tables 2a–2c). No differences were observed in age, CKD stage, comorbidities or concomitant treatments. In patients with PCG (100% of the 48 non-dialysed patients), 90% had a median uricaemia level ≥5 mg/dl during the last 12 months. The reliability study conducted yielded levels of agreement between independent investigators and central observer of 69.9% for DC and 92.4% for tophi.
Differences in clinical variables by joint ultrasound findings.
| Variable | Tophus (n = 16) | No tophus (n = 37) | p-Value |
|---|---|---|---|
| Duration of CKD (years) (median, IQR) | 3.5 (2−6.7) | 7 (3−12) | 0.05 |
| ULT duration (months) (median, IQR) | 22 (12−44) | 39 (29−73) | 0.08 |
| ULT (n, %) | |||
| Allopurinol, 50 mg/day | 0 (0) | 1 (2.7) | |
| Allopurinol, 100 mg/day | 11 (68.8) | 11 (29.7) | 0.05 |
| Allopurinol, 150 mg/day | 5 (31.1) | 4 (10.8) | |
| No ULT | 0 (0) | 21 (56.8) | |
| Average uricaemia (median, IQR) | 7.4 (6.4−8.2) | 8.3 (5.2−8.9) | 0.49 |
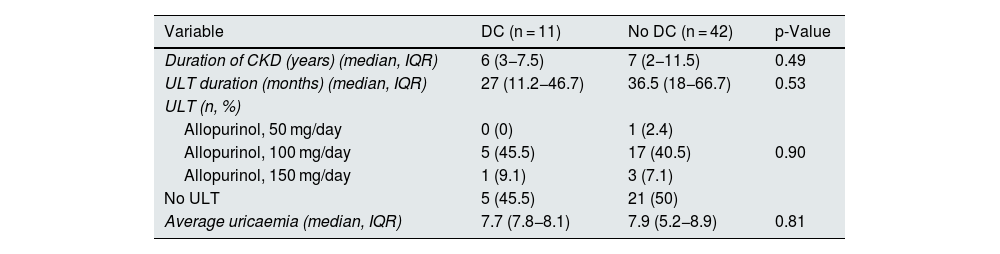
| Variable | DC (n = 11) | No DC (n = 42) | p-Value |
|---|---|---|---|
| Duration of CKD (years) (median, IQR) | 6 (3−7.5) | 7 (2−11.5) | 0.49 |
| ULT duration (months) (median, IQR) | 27 (11.2−46.7) | 36.5 (18−66.7) | 0.53 |
| ULT (n, %) | |||
| Allopurinol, 50 mg/day | 0 (0) | 1 (2.4) | |
| Allopurinol, 100 mg/day | 5 (45.5) | 17 (40.5) | 0.90 |
| Allopurinol, 150 mg/day | 1 (9.1) | 3 (7.1) | |
| No ULT | 5 (45.5) | 21 (50) | |
| Average uricaemia (median, IQR) | 7.7 (7.8−8.1) | 7.9 (5.2−8.9) | 0.81 |
| Variable | Preclinical gout (n = 20) | No preclinical gout (n = 33) | p-Value |
|---|---|---|---|
| Duration of CKD (years) (median, IQR) | 4 (2−7.7) | 7 (3−12) | 0.07 |
| ULT duration (months) (median, IQR) | 24 (13−45) | 39 (28.5−89) | 0.11 |
| ULT (n, %) | |||
| Allopurinol, 50 mg/day | 0 (0) | 1 (3) | |
| Allopurinol, 100 mg/day | 12 (60) | 10 (30.3) | 0.10 |
| Allopurinol, 150 mg/day | 1 (5.5) | 3 (9.1) | |
| No ULT | 7 (35.5) | 19 (57.6) | |
| Average uricaemia (median, IQR) | 7.5 (6.4–8–5) | 8.3 (5.2−9) | 0.45 |
CKD: chronic kidney disease; DC: double contour; ULT: urate-lowering therapy.
This is the first multicentre study focused on the detection of PCG by ultrasound in patients with chronic kidney disease. In our patients, we found a high prevalence of joint ultrasound findings consistent with urate deposits in patients with CKD with no previous symptoms of gout, detecting PCG in four out of 10 patients (tophaceous in three out of 10).
Our data are consistent with the results of studies that have evaluated ultrasound findings consistent with gout in patients with hyperuricaemia detecting a greater presence of DC and tophus in these patients than in patients with normal serum uric acid levels. Pineda et al. also found differences in ultrasound findings of AHU and normal serum uric acid controls (articular tophi 32.7% vs 0%; DC 25% vs 0% in 1MTP; and 17% vs 0% in femoral cartilage);statistical significance was achieved in a study with a larger sample size, lower mean age and greater number of anatomical regions explored, but observing similar levels of SUA in patients with and without tophi (8.13 ± 0.89 vs 8.13 ± 0.99 mg/dl; p = NS), and also finding no correlation between uricaemia and DC (rs −0.06; 95% CI: −0.3 to 0.2).16 Stewart et al. analysed only the 1MTP and found significant differences in DC between patients with AHU and patients with normal serum uric acid levels (36% vs 13%), without detecting tophi in either group, although they did not analyse the relationship between SUA levels and ultrasound findings.21 In another study in patients with no previous symptoms of gout (29% hyperuricaemic), Abhishek et al. did observe higher levels of SUA in those with ultrasound urate deposition (mean difference 0.54 mg/dl [95% CI: 0.12–0.96]), and with ultrasound findings only from SUA ≥ 5 mg/dl (basically identical to that found in our study), with an odds ratio of 1.61 (95% CI: 1.10–2.36) for every 1 mg/dl increase in SUA, although there were more anatomical regions examined and more lesions evaluated (tendon hyperechoic aggregates, in addition to DC and tophus).19 As it can be seen, the lesions and anatomical regions evaluated differ depending on the study analysed, although several authors have recently determined that DC and tophus are the two elementary ultrasound lesions defined by OMERACT with the greatest specificity, precision and positive predictive value for the diagnosis of gout.23 Likewise, a meta-analysis focused on AHU concludes that the evaluation of DC in femoral cartilage and 1MTP, together with tophus in 1MTP, yields the highest prevalence and discriminative power against patients with normal serum uric acid levels.24 Furthermore, the latest EULAR recommendations also advocate DC and tophus as ultrasound lesions to be evaluated in the ultrasound detection of gout.6
Regarding the lack of statistical significance in the differences in ultrasound findings between both groups, this is probably due to the fact that the majority (66.7%) of the patients with normal serum uric acid levels included were receiving ULT (70% of them were defined as hyperuricaemic prior to starting the treatment and some as having elevated serum uric acid levels) and at the time of treatment with ULT, which causes the disappearance of ultrasound lesions (greater for tophi than for DC). In this light, the trend we found towards a higher frequency of tophi in patients with shorter duration of ULT seems plausible, since the amount of urate deposit could be expected to be inversely proportional to the time of uricaemia reduction. It is also possible that patients with a shorter duration of CKD have less eGFR decline and lower uricaemia, receiving ULT less frequently. The use of these drugs is still not widespread in CKD, and are usually administered at low doses, as reported by Jing et al.: 32.5%, falling to 20.7% in patients without gout (despite 62.2% hyperuricaemia in this subgroup), with hyperuricaemia persisting in 47% of those with ULT.9 There is a great deal of variability in the prescription of ULT in CKD owing to doubts regarding its indication, safety or efficacy. All of this could have prognostic implications in CKD (one in four develops gout symptoms; one in three if eGFR <30), and accelerate its progression, with a higher risk of advanced CKD (HR 1.29; 95% CI: 1.23–1.35) and terminal CKD (HR 2.13; 95% CI 1.73–2.61).2,9 Furthermore, urate deposition (mainly tophi) detected by ultrasound has also been associated with a more rapid decline in renal function in patients with gout and CKD (OR 3.56 for ≥10% drop in eGFR at 12 months for cases of tophi; 95% CI: 1.38–9.18).25
One of the limitations of our study is the small sample size, having experienced recruitment difficulties owing to the pandemic. That said, it is an exploratory study and the number of hyperuricaemic patients included is similar to that of other published studies on ultrasound detection of preclinical urate deposits in AHU. Furthermore, this is a clinical practice study, with patients at different CKD stages (including dialysis and excluding stages 1–2) and receiving a wide range of treatments, doses and durations (both ULT and concomitant drugs).
One of the strengths of our study is its multicentre nature, with four evaluators (one for each participating hospital) and an additional centralised reading, with a good degree of agreement found in the interpretation of the images obtained.
ConclusionsWe found a high prevalence of tophi and DC in patients with age 3–5 CKD, predominantly in subjects with median uricaemia ≥5 mg/dl in the last 12 months. Early diagnosis of PCG by musculoskeletal ultrasound and collaboration between nephrology and rheumatology can change the therapeutic approach in CKD, facilitating the improved management of urate-lowering therapy (ULT) in these patients.
EthicsThis study has been approved by the Infanta Leonor-Virgen de la Torre University Hospital Independent Ethics Committee (HUIL-19/00). All patients signed an informed consent form.
FundingThis study has been funded by a grant from the Sociedad de Reumatología de la Comunidad de Madrid [Rheumatology Society of the Community of Madrid] (SORCOM) in collaboration with Merck Sharp & Dohme (MSD).
Special thanks to the Nephrology Departments of the four hospitals participating in this study.
We would like to thank Dr Luis Sala Icardo and Dr Alejandro Prada Ojeda (Rheumatology Department, Torrejón University Hospital) for their support, and Dr María Teresa Navío Marco (Rheumatology Department, Infanta Leonor University Hospital), Dr Alejandro Balsa Criado (Rheumatology Department, La Paz University Hospital) and Dr Mónica Vázquez Díaz (Rheumatology Department, Ramón y Cajal University Hospital) for their trust in the project and the facilities provided to make it possible.