Hyperuricemia has been proposed as an independent factor in the development and progression of chronic kidney disease (CKD). However, the effect of uric acid-lowering therapies on delaying CKD progression is still uncertain. Therefore, this systemic review aims to assess the effect of uric acid-lowering therapies on renal outcomes in pre-dialysis CKD patients.
MethodsPubMed, Cochrane Library, and Lilacs databases were searched until April 24, 2021, for randomized clinical trials of CKD patients on uric acid-lowering treatment with xanthine-oxidase (XO) inhibitors. The weighted mean difference (WMD) or standard mean difference (SMD) with confidence interval (CI) were pooled using a random-effects model.
ResultsAmong 567 studies found, eighteen met the inclusion criteria (n=2463 participants). Compared to the patient's control group, the WMD for the glomerular filtration ratio (GFR) and serum creatinine changes of the treated group was 2.02ml/min/1.73m2 (95%CI 0.41 to 3.63, P=0.014) and −0.19mg/dl (95%CI −0.34 to −0.04, I2=86.2%, P=0.011), respectively. Subgroup analyses showed that the difference in follow-up time and CKD population type in the studies may explain the controversy about the role of uric acid-lowering therapies in CKD progression. The GFR and creatinine outcomes analysis by types of XO inhibitors showed no difference between the control and treated groups. Uric acid-lowering therapies were strongly associated with decreased serum uric acid and urinary protein–creatinine ratio and urinary albumin–creatinine ratio.
ConclusionsThese findings suggest that uric acid-lowering treatment may slow CKD progress and reduce protein and albumin excretion. However, larger and properly powered randomized clinical trials with specific CKD populations are needed to confirm these findings.
La hiperuricemia se ha propuesto como un factor independiente en el desarrollo y la progresión de la enfermedad renal crónica (ERC). Sin embargo, el efecto de las terapias para reducir el ácido úrico en el retraso de la progresión de la ERC aún es incierto. Por lo tanto, esta revisión sistémica tiene como objetivo evaluar el efecto de los tratamientos para reducir el ácido úrico sobre los resultados renales en pacientes con ERC antes de la diálisis.
MétodosSe realizaron búsquedas en las bases de datos de PubMed, Cochrane Library y Lilacs hasta el 24 de abril de 2021 en busca de ensayos clínicos aleatorizados de pacientes con ERC en tratamiento para reducir el ácido úrico con inhibidores de la xantina-oxidasa (XO). La diferencia de medias ponderada (DMP) o la diferencia de medias estándar (DME) con el intervalo de confianza (IC) se agruparon mediante un modelo de efectos aleatorizados.
ResultadosEntre los 567 estudios encontrados, 18 cumplieron los criterios de inclusión (n=2.463 participantes). En comparación con los pacientes del grupo control, la DMP para la tasa de filtración glomerular (TFG) y los cambios en la creatinina sérica del grupo tratado fueron de 2,02ml/min/1,73m2 (IC del 95%: 0,41 a 3,63, P=0,014) y −0,19mg/dl (IC del 95%: −0,34 a −0,04, I2=86,2%, P=0,011), respectivamente. Los análisis de subgrupos mostraron que la diferencia en el tiempo de seguimiento y el tipo de población con ERC en los estudios puede explicar la controversia sobre el papel de las terapias para reducir el ácido úrico en la progresión de la ERC. El análisis de resultados de TFG y de creatinina por tipos de inhibidores de la XO no mostró diferencias entre el grupo control y el grupo tratado. Las terapias para reducir el ácido úrico se asociaron fuertemente con una disminución del ácido úrico sérico y de la relación proteína-creatinina urinaria y la relación albúmina-creatinina urinaria.
ConclusiónEstos hallazgos sugieren que el tratamiento para reducir el ácido úrico puede retrasar el progreso de la ERC y reducir la excreción de proteínas y de albúmina. Sin embargo, se necesitan ensayos clínicos aleatorizados más grandes y con el poder estadístico adecuado con una población específica con ERC para confirmar estos hallazgos.
Clinically significant chronic kidney disease (CKD) is defined as a glomerular filtration rate (GFR) below 60ml/min/1.73m2 present for >3 months.1 It is estimated that CKD affects 15% of the adult population in the United States, and between 8% and 16% of the population worldwide, which is a challenge for health services.1–3
The kidneys are responsible for excreting two-thirds of uric acid circulating in the blood. Thereby, hyperuricemia is among CKD complications.4 The prevalence of hyperuricemia is 20.1% in the United States general population and it has increased over several decades, reaching 38% among CKD patients.5,6
Hyperuricemia, defined as a plasma uric acid levels >6.8mg/dl,7 is the primary precursor of gout and it is involved in the pathogenesis of different diseases, such as hypertension, obesity, hypercholesterolemia, atherosclerosis, metabolic syndrome, and chronic heart failure.8
The association of hyperuricemia with the risk of progression of CKD has been shown in some studies,9–12 which has led to clinical trials to assess the association between acid uric-lowering therapy and CDK progression with diverging results. Thus, this study aimed to perform a systematic review to evaluate the association of urate-lowering treatments and CKD progression.
MethodsThis systematic review was conducted according to the Preferred Items guidelines for Reporting for Systematic Reviews and Meta-Analysis (PRISMA). This study was not registered.
Search strategyThis study is a systematic review of randomized controlled trials that consisted of a search in the databases: PubMed, Lilacs and, Cochrane Library. Studies published until April 24, 2021, were included. The following keywords were used as search terms: ‘chronic kidney disease’, ‘chronic renal insufficiency’, ‘CKD’, ‘uric acid’, ‘uric acid lowering therapy’, ‘urate-lowering therapy’. The language of the searches was limited to Portuguese, English and Spanish. The references of all retrieved articles were also manually selected.
Study selectionTwo independent authors screened the study. Disagreements were resolved through discussion with a third author. Titles and abstracts of retrieved articles were revised, followed by screening. The studies were included considering the following PICO criteria: (1) adult patients with CKD, except End-stage Renal Disease (ESRD). The CKD was defined according to the KDIGO guidelines1; (2) patients on uric acid-lowering treatment with xanthine oxidase (XO) inhibitors; (3) patients who did not use uric acid-lowering treatment as the control group; (4) and reported change in GFR, urinary protein–creatinine ratio (uPCR), or urinary albumin–creatinine ratio (uACR). Only randomized clinical trials were included.
Data extractionTwo independent authors carried out data extraction according to a pre-designed data collection form. Disagreements were discussed with a third author. The extracted data included author's name, year of publication, study design, country of origin, demographic characteristics (age, sex, and sample size), type of treatment for hyperuricemia, and duration from the follow-up, initial kidney function, change in kidney function (reported as GFR or serum creatinine concentration or creatinine clearance) from baseline to the end of follow-up.
Risk of biasTwo authors independently assessed the quality of studies according to the Cochrane guidelines.13 Any disagreements were resolved through discussion with a third author. Five domains were assessed: (1) bias arising from the randomization process; (2) bias due to deviations from the intended interventions; (3) bias due to missing outcome data; (4) bias in the measurement of the outcome; (5) bias in the selection of the reported results.
Results evaluationThe primary outcome was the effect of treatment on the GFR difference. The secondary analyses focused on the effect of treatment on the serum creatinine level difference, serum uric acid level difference, proteinuria level difference and, uPCR or uACR difference.
Statistical analysisWeighted mean difference (WMD) and standardized mean difference (SMD) were calculated with a 95% confidence interval (CI). For GFR, serum creatinine, serum uric acid was analyzed utilizing WMD, while the comparative analysis of uPCR and uACR between groups was performed together utilizing SMD, according to Higgins et al.14,15 The Cochran's Q test and I2 were used to assess the statistical significance and the degree of heterogeneity between studies, respectively.
The P≤0.05 for the Q test represented a significance difference between the groups, and an I2≥50% statistic revealed substantial heterogeneity. If the heterogeneity test was not significant, the analyses were performed using a fixed-effects model; otherwise, a random-effects model was used. We also conducted a sensitivity analysis by removing each study from the meta-analysis for the presence of heterogeneity. When not reported, the standard deviations (SD) in the studies were calculated according to the Cochrane Handbook's equations for Systemic Review.14 Finally, the publication bias was examined by the Egger test and funnel plot. All analyses were performed with Stata/SE v.14.1 software (StataCorpLP, USA).
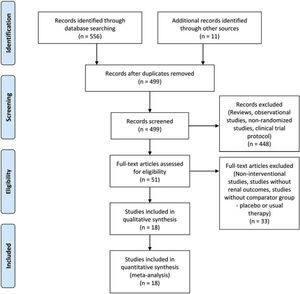
ResultsStudy selection and characteristicsFive hundred sixty-seven studies were identified in the search. Sixty-eight were excluded because they were duplicated. Reviews, observational studies, non-randomized studies, and clinical trial protocols were excluded. From the remaining studies, eighteen randomized clinical trials met the inclusion criteria and were included in the meta-analysis (Fig. 1), totalizing 2463 patients. The investigated therapies found were allopurinol in nine studies,16–24 seven studies investigated febuxostat,25–31 one study investigated febuxostat plus verinurad,32 and one trial investigated the effect of topiroxostat on CKD progression.33 The basic studies characteristics were shown in Table 1.
Characteristics of studies selected.
| Authors | Year | Country | Study design | Uric acid-lowering therapy | Population | Sample size | Age mean (SD) | Treatment group | Control group | Follow up |
|---|---|---|---|---|---|---|---|---|---|---|
| Badve et al. | 2020 | Australia and New Zealand | Randomized, multicenter, double-blind, placebo-controlled trial | Allopurinol 100mg versus Placebo | Adults with stage 3 or 4 CKD with a urinary albumin:creatinine ratio of 265 or higher or a decrease in eGFR of at least 3.0ml/min per 1.73m2 in the preceding 12 months. | 363 | Treatment group62.3±12.6Control group62.6±12.9 | 182 | 181 | 2 years |
| Beddhu et al. | 2016 | USA | Randomized, double-blind, placebo-controlled trial | Febuxostat 40mg versus Placebo | Patients with type 2 DM, CKD stage 3 and asymptomatic hyperuricemia | 76 | Treatment group67±10Control group68±11 | 37 | 39 | 24 weeks |
| Doria et al. | 2020 | USA, Canada, and Denmark | Randomized, multicenter, double-blind, placebo-controlled trial | Allopurinol 100–400mg/day according to GFR | Patients with type 1 DM and nephropathy | 530 | Treatment group50.4±11.2Control group51.8±10.6 | 267 | 263 | 3 years |
| Goicoechea et al. | 2010 | Spain | Randomized, open-label, controlled trial | Allopurinol 100mg versus usual therapy | Patients with GFR lower than 60ml/min | 113 | Treatment group72.1±7.9Control group71.4±9.5 | 57 | 56 | 2 years |
| Golmohammadi et al. | 2017 | Iran | Randomized, placebo-controlled trial | Allopurinol 100mg versus Placebo | Adults with stage 3 or 4 CKD and asymptomatic hyperuricemia | 177 | No reported | 77 | 100 | 12 months |
| Hosoya et al. | 2014 | Japan | Randomized, multicenter, double-blind, placebo-controlled trial | Topiroxostat 160mg versus Placebo | Adults with stage 3 or 4 CKD and asymptomatic hyperuricemia | 122 | Treatment group62.5±8.8Control group64.6±8.1 | 62 | 60 | 22 weeks |
| Kao et al. | 2011 | UK | Randomized, multicenter, double-blind, placebo-controlled trial | Allopurinol 300mg versus Placebo | Patients with stage 3 CKD and LVH | 53 | Treatment group70.6±6.9Control group73.7±5.3 | 27 | 26 | 9 months |
| Kimura et al. | 2018 | Japan | Randomized, multicenter, double-blind, placebo-controlled trial | Febuxostat 40mg versus Placebo | Adults with stage 3 CKD and asymptomatic hyperuricemia | 441 | Treatment group65.3±11.8Control group65.4±12.3 | 219 | 222 | 108 weeks |
| Mukri et al. | 2018 | Malaysia | Randomized, open-label, controlled trial | Febuxostat 40mg versus usual therapy | CKD stage 3 and 4 patients with diabetic nephropathy | 93 | Treatment group64.0±10.0Control group67.0±6.0 | 47 | 46 | 6 months |
| Momeni et al. | 2010 | Iran | Randomized, double-blind, placebo-controlled trial | Allopurinol 100mg versus Placebo | Patients with type 2 DM and nephropathy (proteinuria greater than 500mg/24h) and GFR>25ml/min | 40 | Treatment group56.3±10.6Control group59.1±10.6 | 20 | 20 | 4 months |
| Perrenoud et al. | 2020 | United States | Randomized, double-blind, placebo-controlled trial | Allopurinol 300mg versus Placebo | Patients with stage 3 CKD and asymptomatic hyperuricemia | 69 | Treatment group59.0±12.0Control group58.0±9.0 | 33 | 36 | 12 weeks |
| Saag et al. | 2016 | USA | Randomized, multicenter, double-blind, placebo-controlled trial | Febuxostat 60mg/day versus Placebo | Patients with gout and GFR between 15 to 50ml/min/1.73m2 | 64 | Treatment group67.3 6 ±11.1Control group66.3 6 12.1 | 32 | 32 | 12 months |
| Shi et al. | 2011 | China | Randomized, open-label, controlled trial | Allopurinol 100–300mg/day according to the levels of Scr and UA versus usual therapy | Patients with IgA nephropathy, proteinuria between 0.15 and 2.0g/24h, serum albumin level >13.5g/dl, Scr level <3mg/dl and asymptomatic hyperuricemia | 40 | Treatment group39.7±10.0Control group40.1±10.8 | 20 | 20 | 6 months |
| Sircar et al. | 2015 | India | Randomized, double-blind, placebo-controlled trial | Febuxostat 40mg versus placebo | Adults with stage 3 or 4 CKD and asymptomatic hyperuricemia | 93 | Treatment group56.2±10.9Control group58.4±14.5 | 45 | 48 | 6 months |
| Siu et al. | 2006 | China | Randomized, open-label, controlled trial | Allopurinol 100–200mg/day according to the levels of Scr and UA versus usual therapy | Patients with presence of renal disease (proteinuria greater than 0.5g and/or an elevated Scr level greater than 1.35mg/dl) and asymptomatic hyperuricemia without renal stones, and advanced chronic kidney disease | 51 | Treatment group47.7±12.9Control group48.8±16.8 | 25 | 26 | 12 months |
| Stack et al | 2021 | UK | Randomized, multicenter, double-blind, placebo-controlled trial | Verinurad 9mg+Febuxostat 80mg versus placebo | Adults with Type 2 DM, serum UA≥6.0mg/dl, GFR)≥30ml/min/1.73m2, and uACR of 30–3500mg/g | 60 | Treatment group62.0±9.5Control group60.9±12.2 | 32 | 28 | 24 weeks |
| Tanaka et al. | 2015 | Japan | Randomized, open-label, controlled trial | Febuxostat 10–40mg/day according to the levels of UA versus usual therapy | Patients with stage 3 CKD and asymptomatic hyperuricemia | 40 | Treatment group70.1±9.5Control group66.1±7.0 | 21 | 19 | 12 weeks |
| Wen et al. | 2020 | China | Randomized, open-label, controlled trial | Febuxostat 10–40mg/day according to the levels of UA versus usual therapy | Patients with stage 3 CKD, type 2 DM and asymptomatic hyperuricemia | 38 | Treatment group58.73±11.50Control group57.46±10.96 | 18 | 20 | 24 weeks |
CKD: chronic kidney disease, DM: diabetes mellitus, GFR: glomerular filtration rate, LVH: left ventricular hypertrophy, UA: uric acid, uACR: urinary albumin–creatinine ratio, Scr: serum creatinine.
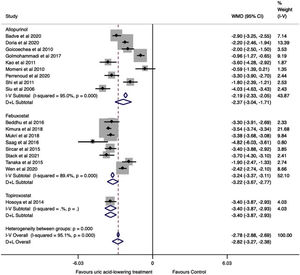
As shown in Fig. 2, there was a significant reduction in serum uric acid in the treatment group compared to the control group (WMD=−2.82mg/dl, 95%CI −3.27 to −2.36, I2=95.1%, P<0.001). In the analysis by drugs, allopurinol studies revealed a difference in serum uric acid between the groups of −2.37mg/dl (95%CI −3.04 to −1.71, I2=95.0%, P<0.001); the difference in serum uric acid in the febuxostat studies was −3.22mg/dl (95%CI −3.67 to −2.77, I2=89.4%, P<0.001) in favor of treatment group.
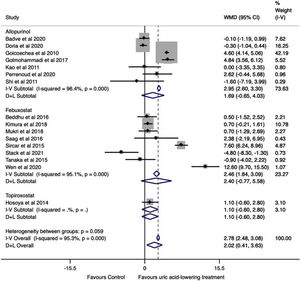
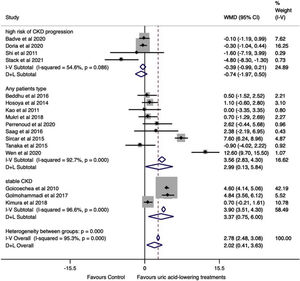
Effect of uric acid-lowering treatment on GFRThe data were extracted and pooled from sixteen studies. Seven trials studied allopurinol treatment, eight febuxostat treatment, and one topiroxostat. Compared with the control group, the pooled estimate showed a statistically significant change in GFR in the uric acid-lowering treatment group (WMD=2.02ml/min/1.73m2, 95%CI 0.41 to 3.63, I2=95.3%, P=0.014). In the analysis by drugs, no difference was found in the change in GFR between groups (allopurinol with WMD=1.69ml/min/1.73m2, 95%CI −0.65 to 4.03, I2=96.4% with P=0.157, febuxostat with WMD=2.40ml/min/1.73m2 (95%CI −0.77 to 5.58, I2=95.1%, P=0.138), and topiroxostat with WMD=1.10ml/min/1.73m2 (95%CI −0.60 to 2.80, P=0.205) (Fig. 3).
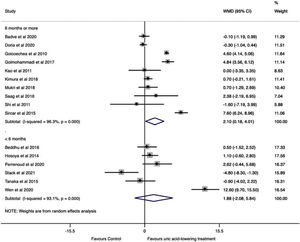
A subgroup analysis by follow-up time was performed. Studies with six months or more of follow-up showed a significant change in GFR in the uric acid-lowering treatment group (WMD=2.10ml/min/1.73m2, 95%CI 0.18 to 4.02, I2=96.3%, P=0.032), while in studies with less than six months of follow-up no difference was found (WMD=1.88ml/min/1.73m2, 95%CI −2.08 to 5.84, I2=93.1%, P=0.351) (Fig. 4).
Another subgroup analysis according to the patient's risk of CKD progression was performed (Fig. 5). Studies pooled from patients with a low risk of CKD progression showed a change in GFR favoring of the treatment group (WMD=3.37ml/min/1.73m2, 95%CI 0.75 to 6.00, I2=96.6%, P<0.001). The same occurred for studies that included subjects, regardless the patients’ CKD stage (WMD=2.99ml/min/1.73m2, 95%CI 0.13 to 5.84, I2=92.7%, P<0.001). On the other way, analysis of studies that included patients with a higher risk of CKD progression revealed no difference between treated and control groups (WMD=−0.74ml/min/1.73m2, 95%CI −1.97 to 0.50, I2=54.6%, P=0.208).
Effect of uric acid-lowering treatment on serum creatinineThe data were extracted from seven studies. Three trials studied allopurinol treatment and four febuxostat treatment. The pooled for the change between baseline and final serum creatinine levels showed a decrease in uric acid lowering treatment group of −0.19mg/dl (95%CI −0.34 to −0.04, I2=86.2%, P=0.011) than the control group, indicating a significant benefit in patients with urate-lowering treatments (Fig. S1).
In the subgroup analysis, the treatment with allopurinol showed no difference in creatinine change between groups (WMD=−0.27mg/dl, 95%CI −0.59 to 0.06, I2=80.5%, P=0.112). The result was similar for febuxostat treatment (WMD=−0.15mg/dl, 95%CI −0.41 to 0.10, I2=90.3%, P=0.236).
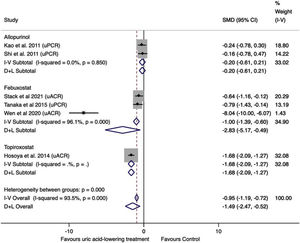
Effect of uric acid-lowering treatment on proteinuria (uPCR and uACR)The data were extracted from six trials. Of these, two studied allopurinol treatment, three febuxostat treatment, and one topiroxostat treatment. Three studies reported uPCR, and three studies reported uACR. The uACR dates were analyzed with uPCR dates using SMD.14,15 SMD is the mean difference expressed in units of SD. Values up to ±0.5 are considered small, and values >±0.8 are considered large.15 Combining the six studies, the result was −1.49 (95%CI −2.47 to −0.52, I2=93.5%, P=0.003), suggesting a significant and strong decrease in proteinuria in the treatment group compared to the control group (Fig. 6).
In subgroup analysis, the pooled allopurinol studies showed no difference between groups (SMD=−0.20, 95%CI −0.61 to 0.21, P=0.333), without significant heterogeneity (I2=0.0%). The pooled febuxostat studies showed a decrease of −2.83 (95%CI −5.17 to −0.49, I2=96.1%, P=0.018) in proteinuria in the treatment group compared to the control group.
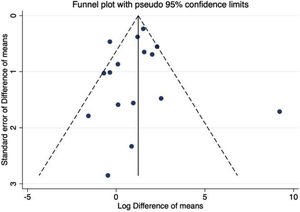
Sensitivity analyses, assessment of heterogeneity and publication biasSensitivity analyses were performed by excluding one study at a time for GFR outcome, and the values observed from the analysis did not change the findings (Table S1). The estimated bias coefficient results were from 0.235 to 1.078, giving a P-value >0.05 for all analyses. Therefore, the tests provide weak evidence for the presence of publication bias. A funnel plot was performed but failed to detect possible small study effects (Fig. 7).
Quality assessment of selected studies for meta-analysesAmong the 18 studies selected for the meta-analyses, six trials18,20,22,23,29,31 were considered as high risk of bias, six17–19,27,30,32 as some concerns, and six trials16,24–26,33,34 as low risk of bias. Eleven trials were randomized, double-blind, placebo-controlled trial, one randomized, single-blind placebo-controlled trial, and six trials were randomized, open-label, controlled studies. The quality assessments of the studies included in the meta-analysis are shown in Fig. S2.
DiscussionThis study is a systematic review followed by a meta-analysis evaluating a possible association between XO inhibitors as uric acid-lowering therapies and the progression of CKD with the largest number of patients involved.
This study showed a significant difference in the change of GFR of the treated group compared to the control group, combining all therapies. Likewise, CKD patients who received uric acid-lowering treatment had a substantial decrease in serum uric acid, serum creatinine and proteinuria. However, there was no difference in the GFR and creatinine between the groups when analyzed by individual therapies. For uACR and uPCR outcomes, febuxostat and topiroxostat treatment decreased proteinuria, while the allopurinol treatment group showed did not difference compared to the control group.
Some hypotheses will be discussed below that may explain our results. Due to the insidious nature of CKD, one hypothesis is the follow-up time required to demonstrate the benefit in GFR. For example, urate promotes kidney damage with long-term exposure. Thus, the follow-up may be an essential factor in the trials that studied the association between uric acid-lowering and renal outcomes.24,35 Sato et al. showed that among trials in which patients in the control group experienced progressive deterioration of kidney function was typically in studies with 6 months to 2 years of follow-up.52
For this reason, we performed a subgroup analysis of the change in GFR by follow-up time. Studies with 6 months or more of follow-up showed a significant difference in GFR of the treated patients compared to the control groups, while the results of pooled studies with less than 6 months no difference were found between the treatment and control groups.
The sample size of the selected studies could also help to explain the divergence of the results between the analyses of individual and pooled therapies. Serdar et al. report that for minor effects size or if the variability of this effect on the population for extensive, it is necessary to increase the sample size.36 When we separated the studies by drugs, this led to a reduction in the sample size in subgroups, and this may mean that the benefit of uric acid-lowering therapy in GFR exists, but the effect is small.
Another vital aspect being discussed is the result of the analysis by CKD stage of the patients included in the studies. When the studies were pooled according to the risk of CKD progression, it was observed that uric acid-lowering therapy attenuated the decline in the GFR in low-risk CKD patients, but not in patients with an elevated risk of CKD progression. One hypothesis to explain the difference in the effect of uric acid-lowering treatments in the patients at low and high risk of CKD progression is that hyperuricemia may be just one of the factors that cooperate for the progression of CKD, and uric acid-lowering therapies may not be enough to attenuate the disease in the presence of the other causes that may be intensified.
Recent studies with allopurinol found no benefit in reducing CKD progression with the use of hypoglycemic therapies, which generated scepticism on the part of the nephrological community.16,24,37 However, the Preventing Early Renal Loss in Diabetes (PERL) and the Controlled Trial of Slowing of Kidney Disease Progression from the Inhibition of Xanthine Oxidase (CKD-FIX) studies recruited subjects irrespective of their serum uric acid concentration. For example, PERL study included patients with serum urate starting at 4.5mg/dl, and the mean serum urate in both treated and placebo groups was 6.1mg/dl. The association between serum uric acid levels and CKD development is not linear but increases exponentially for values of serum uric acid >7mg/dl.38
In this line of reasoning, also in a recently published cohort single-center prospective study involving 411 hypertensive, non-diabetic, Chinese patients with a median follow up of 4 years, Huang et al.39 found that only uric acid level above 7.5mg/dl was independently associated with time-dependent reduction of patients’ GFR and the authors suggested that this cut-off value is of clinical importance. Piani et al.38 address that the controversy between studies on uric acid-lowering therapy and CKD progression could be explained by selection bias due to the heterogeneity of the hyperuricemic population, which could also explain the persistent heterogeneity in our analysis.
Lastly, CKD-FIX study involved stage 3 and 4 CKD patients with evidence of a reduction of at least 3ml/min/1.73m2 in the previous year. The follow up was two years. Due to a decision made by the trial steering committee, only 60% of the intended number of patients were enrolled, which may have affected the results.
Regarding uACR and uPCR, sensitive markers of kidney damage, they may reflect that proteinúria can present a faster response to therapy and risk of CKD progression than the GFR measurement.40 Our study showed, in the uACR/uPCR analysis, that there was a benefit in the uric acid-lowering therapy group. Proteinuria and albuminúria are known to be independent predictors of CDK progression. Therefore, reducing albuminuria and proteinuria is a target for renoprotection in patients with CKD. Zeeuw et al.41 showed that for every 50% reduction in proteinuria in patients with type 2 diabetic nephropathy, there was a 45% reduction in ESRD risk.
Some hypotheses have been described linking hyperuricemia with CKD progression. Many studies have suggested that hyperuricemia is an independent risk factor for renal disease and has a role in the pathogenesis of kidney injury in CKD patients. Thus, it is possible that uric acid-lowering treatment may delay the progression of CKD, at least in patients with hyperuricemia and low risk of CKD progression.
Hyperuricemia patients may have the deposition of monosodium urate crystals in the tubules or interstitium in the kidney that leads to chronic inflammation and tubular damage.42 Although urate crystal deposition in the kidney might be expected to be higher in patients with gout, people with asymptomatic hyperuricemia may have silent crystal deposition in the kidney.38
Mild hyperuricemia also has been associated with an increased risk of developing hypertension, diabetes and metabolic syndrome, factors that contribute to the development and progression of renal dysfunction.43 Hyperuricemia has also been associated with endothelial dysfunction, resulting in thickening of the afferent arterioles and decreased vasodilation, factors involved in the kidney injury.44,45 Tsuruta et al.46 showed in their trial that hemodialysis patients with hyperuricemia treated with febuxostat had a significant reduction in their endothelial dysfunction and oxidative stress.
Even a slight increase in serum uric acid levels may cause mitochondrial dysfunction, activate the renin–angiotensin–aldosterone-system (RAAS) and promote salt sensitivity.43 Besides, high serum uric acid levels are linked with the proliferation of vascular smooth muscle, which causes an increase in glomerular hydrostatic pressure.47 Furthermore, uric acid may stimulate the synthesis of IL-6, and tumor necrosis factor α, through contributing to the development of vascular diseases and CKD.48,49
This review selected studies contained 3 different therapies: allopurinol, febuxostat and topiroxostat. These drugs are XO inhibitors.50–53 The xanthine oxidase has a role in catalyzing hypoxanthine's oxidation to xanthine and xanthine to uric acid, which is why these drugs promote the reduction of uric acid synthesis.54 Additionally, reactions promoted by xanthine oxidase generate reactive oxygen species, superoxide anion and hydrogen peroxide, which may cooperate for oxidative stress, a common event in patients with CKD and considered to be an important pathogenic mechanism to the progression of CKD.55,56 Therefore, uric acid-lowering may slow CKD progress by inhibiting reactive oxygen species and suppressing RAAS activity, leading to increased glomerular perfusion.50 Yang et al.57 showed benefits in oxidative stress markers with allopurinol therapy in rabbits with diabetes.
The findings of this systematic review corroborate with other studies. Liu et al.58 showed that the risk of worsening kidney function, ESRD or death was significantly decreased in the allopurinol group compared to the control group. Kanji et al.59 evaluated all urate-lowering therapies on CKD progression, and the GFR mean difference founding favored treatment.
In the analysis of the effect of uric acid-lowering therapy in changes in serum uric acid after treatment, our study showed that patients treated with febuxostat reduced their serum uric acid to a significantly lower level than patients treated with allopurinol (−3.22mg/dl versus −2.37mg/dl). Sezai et al.60 found in cardiac surgery patients with hyperuricemia that febuxostat reduced uric acid earlier than allopurinol, which had a more substantial renoprotective effect than allopurinol, and had superior antioxidant and anti-inflammatory properties.
This study has several strengths. To our knowledge, this is the first meta-analysis that assessed uACR and uPCR as outcomes and includes topiroxostat treatment. This study informs physicians regarding the effect of XO inhibitors therapy on CKD progression. Despite the few published clinical trials, the studies selected in this systematic review added up 2463 patients, with the most significant number of patients involved.
Some limitations of our study were the small number of randomized trials on the use of topiroxostat in patients with CKD patients, different follow-up times between the studies, three studies did not report on the use of renin-angiotensin system inhibitors,18,27,31 studies performed without blinding,17,22,23,27,28,31 and substantial heterogeneity. Differences in population characteristics, study designs, sample sizes, and follow-up time may have contributed to high heterogeneity.
ConclusionThese results suggest that uric acid-lowering treatment with XO inhibitors may slow the progress of CKD and reduce proteinuria. Nevertheless, these findings should be interpreted considering the study limitations. Large and powered randomized clinical trials, with adequate follow-up time and with specific CKD populations (by the risk of CKD progression and with hyperuricemia), are needed to assess the effects of uric acid-lowering therapies in GFR and proteinuria of CKD patients.
Conflict of interestThe authors declare that they have no conflict of interest.