In kidney transplant (KT) recipients diabetes mellitus (DM) are associated with an increased mortality and a poorer graft survival. Glucagon-like peptide 1 receptor agonists (GLP1-RA) have demonstrated cardiovascular and renal benefits in the general population. However, there is lacking evidence in KT recipients.
ObjectiveTo analyze the efficacy and safety of glucagon-like peptide 1 receptor GLP1-RA in a cohort of KT recipients.
MethodsMulticenter retrospective cohort study of KT patients with DM who started subcutaneous GLP1-RA in 3 hospitals in the province of Cádiz between February 2016 and July 2022. Estimated glomerular filtration rate (eGFR), proteinuria, and weight at baseline and after 6 and 12 months were collected. We analyzed glycemic control, blood pressure, lipid profile, and doses and trough levels of tacrolimus. We document episodes of acute rejection (AR), de novo donor-specific antibodies (dnDSA), and adverse effects.
ResultsDuring this period, 96 KT with DM started treatment with GLP1-RA, of which 84 had a minimum follow-up of 6 months and 61 were followed for 12 months. A significant reduction was observed in proteinuria (−19.1 mg/g, p = 0.000; −46.6 mg/g, p = 0.000), weight (−3.6 kg, p = 0.000; −3.6 kg, p = 0.000), glycosylated hemoglobin (−0.7%, p = 0.000; −0.9%, p = 0.000), systolic blood pressure (−7.5 mmHg, p = 0.013; −7.3 mmHg, p = 0.004), total cholesterol (−11.5 mg/dL, p = 0.001; −15.6 mg/dl, p = 0.002) and LDL cholesterol (−9.2 mg/dl, p = 0.002; −16.8 mg/dl, p = 0.000) at 6 months and 1 year of follow-up. The eGFR remained stable and the dose and trough levels of tacrolimus did not change. No episodes of AR or development of dnDSA were observed during follow-up.
ConclusionsGLP1-RA in KT patients can be a safe and effective option for the management of DM in KT.
En los pacientes receptores de trasplante renal (TR) la diabetes mellitus (DM) se relaciona con una mayor mortalidad y menor supervivencia del injerto. Los agonistas del receptor del péptido 1 similar al glucagón (ar-GLP1) han demostrado beneficios cardiovasculares y renales en la población general. Sin embargo, su evidencia en pacientes TR es limitada.
ObjetivoAnalizar la eficacia y seguridad de los ar-GLP1 en una cohorte de pacientes TR.
MétodosEstudio de cohortes retrospectivo multicéntrico de los pacientes TR con DM que iniciaron ar-GLP1 de administración subcutánea en 3 hospitales de la provincia de Cádiz entre febrero de 2016 y julio de 2022. Se recogió filtrado glomerular estimado (FGe), proteinuria y peso al inicio del tratamiento y tras 6 y 12 meses. Analizamos control glucémico, tensión arterial, perfil lipídico y niveles valle y dosis de tacrolimus. Documentamos episodios de rechazo agudo (RA), anticuerpos donantes específicos de novo (DSAn) y efectos adversos.
ResultadosEn este periodo 96 TR con DM iniciaron tratamiento con ar-GLP1, de los cuales 84 cumplieron el seguimiento mínimo de 6 meses y 61 pacientes de 1 año. Se observó una reducción significativa de la proteinuria (−19.1 mg/g, p = 0.000; −46.6 mg/g, p = 0.000), peso (−3.6 kg, p = 0.000; −3.6 kg, p = 0.000), hemoglobina glicosilada (−0.7 %, p = 0.000; −0.9%, p = 0.000), tensión arterial sistólica (−7.5 mmHg, p = 0.013; −7.3 mmHg, p = 0.004), colesterol total (−11.5 mg/dL, p = 0.001; −15.6 mg/dl, p = 0.002) y LDL colesterol (− 9.2 mg/dl, p = 0.002; −16.8 mg/dl, p = 0.000) a los 6 meses y al año de seguimiento. El FGe se mantuvo estable y no se modificó ni la dosis ni los niveles valle de tacrolimus. No se objetivaron episodios de RA ni desarrollo de DSAn durante el seguimiento.
ConclusionesLos ar-GLP1 en pacientes TR demuestran que puede ser una opción segura y eficaz para el manejo de la DM en TR.
Diabetes mellitus (DM) is a complication after kidney transplantation (KT), with a prevalence of approximately 40%. In addition, 10–20% of non-diabetic KT recipients develop post-transplant diabetes mellitus (PTDM). Both pre-existing DM and PTDM are associated with higher mortality of the recipient and affects negatively renal graft survival,1–3 owing to which there is a need to modify risk factors in this population.
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are a class of anti-diabetic drugs that have demonstrated cardiovascular and renal benefits; they reduce proteinuria and slow the decline of glomerular filtration rate (eGFR).4,5 They also promote weight loss, since they delay gastric emptying and promote satiety. Furthermore, they carry a lower risk of hypoglycemia as they induce glucose-dependent insulin secretion.4,5 Their metabolism does not involve cytochrome P450 enzymes or transporter-mediated drug–drug interactions. Nonetheless, it is suggested that their role in gastric emptying could affect the absorption of certain drugs.4,5
However, experience with GLP-1 RAs in KT is limited.6–11 We have previously reported the results of our experience with a small group of KT recipients.12 The present study aims to analyze treatment results with GLP-1 RAs in a larger cohort with longer follow-ups to evaluate the effectiveness and safety of these drugs in diabetic KT recipients.
MethodsThis was a multicentre retrospective cohort study of KT recipients with pre-existing DM or PTDM who commenced treatment with subcutaneously administered GLP-1 RAs between February 2016 and July 2022. The prescription criteria were based on recommendations from the American Diabetes Association.13 The use of GLP-1 RAs was preferred over sodium-glucose cotransporter type 2 inhibitors (SGLT2i) in subjects with obesity (body mass index [BMI] >30 kg/m2). The three hospitals that conducted follow-ups on KT recipients in the province of Cadiz participated (Puerta del Mar, Jerez de la Frontera and Puerto Real Hospitals). In all subjects prescribed a GLP-1 RA, monthly check-ups were performed until the maximum tolerated dose of the drug was reached, to monitor immunosuppressant levels and identify adverse effects. Subsequently, the check-ups were spaced out according to the needs of each subject and time post-KT. All subjects included had a minimum follow-up of six months after starting the drug. Given the efficacy of these drugs shown by other groups, we did not propose a control group and we started treatment in those subjects in whom it was considered that GLP-1 RAs could provide special benefits, such as obese diabetic subjects.
Clinical and analytical data were collected at baseline, and at 6 and 12 months after treatment initiation. The eGFR was measured using the Modification of Diet in Renal Disease (MDRD) equation.14 Proteinuria was quantified using the albumin/creatinine ratio (ACR) in a first-morning urine sample. Blood pressure (BP) was measured by a nurse using an oscillometric device (Welch Allyn Spot Vital Signs LXi, Soma Technology Inc., USA) with the subject seated, before entering the doctor's office and at least five minutes after arrival. BP was calculated as an average of three measurements taken five minutes apart. We documented episodes of acute rejection (AR), development of de novo donor-specific antibodies (dnDSA) and occurrence of adverse effects. dnDSA were requested prospectively annually in all subjects or before any episode of renal function deterioration. We collected type and dose of GLP-1 RAs and other anti-diabetic, lipid-lowering, anti-hypertensive and immunosuppressive drugs; as well as tacrolimus trough levels, dose and C/D ratio (pre-dose tacrolimus blood levels [ng/mL]/daily dose of tacrolimus [mg]) in each period analyzed.
Statistical analysisContinuous variables are presented as mean and standard deviation (SD) or median and interquartile range (IQR), as appropriate, and categorical variables as absolute value and percentage. Continuous variables were compared using parametric tests (Student's t-test for paired data) and non-parametric tests (Wilcoxon test for paired data and the Kruskal-Wallis test for three or more independent groups). The normality of the samples was analyzed using the Kolmogorov-Smirnov test. For categorical variables, significant differences were assessed using the McNemar test for comparisons between visits. Subjects who failed to complete the minimum six-month follow-up after starting the drug were excluded from the analysis. Once this period had passed, all subjects were included, even if the drug was subsequently discontinued for any reason. Significance was set at p < 0.05. The statistical package SPSS version 26.0 was used to analyze the variables studied (IBM Corp., Armonk, NY, USA).
ResultsDuring the study period, 96 subjects started treatment with GLP-1 RA. Baseline characteristics are shown in Table 1. The majority were male (56.2%) with a mean age of 61.6 ± 9.7 years. In total, 53 subjects (55.2%) had pre-existing DM, and the rest developed PTDM. Semaglutide was the most prescribed GLP-1 RA (66.7%), followed by liraglutide (20.8%) and dulaglutide (12.5%). Some 52 subjects (54.2%) reached the maximum recommended dose of the drug. Finally, 84 KT recipients reached a minimum follow-up of six months after the start of treatment and, of these, 61 completed 12 months of follow-up (Fig. 1). The study variables compared at six and 12 months are shown in Table 2 and Fig. 2. The analysis of the main variables was also performed by comparing the three types of GLP-1 RA (semaglutide, liraglutide, and dulaglutide) that our subjects received, with no significant differences observed between them being in any of the variables analyzed (Table 3).
Clinical and demographic characteristics of kidney transplant (KT) recipients included at the start of treatment with GLP-1 RA.
| Characteristics | Value |
|---|---|
| Male, n (%) | 54 (56.2) |
| Age (years), mean (SD) | 61.6 (9.7) |
| Weight (kg), mean (SD) | 95.0 (15.4) |
| BMI (kg/m2), mean (SD) | 35.8 (4.8) |
| HTN, n (%) | 95 (98.9) |
| DLP, n (%) | 95 (98.9) |
| SBP (mmHg), mean (SD) | 147.6 (19.8) |
| DBP (mmHg), mean (SD) | 77.7 (9.6) |
| Pre-existing DM, n (%) | 53 (55.2) |
| PTDM, n (%) | 42 (43.7) |
| Heart failure, n (%) | 14 (14.6) |
| Ischaemic heart disease, n (%) | 23 (23.9) |
| Obliterative arteriopathy, n (%) | 14 (14.6) |
| Diabetic retinopathy, n (%) | 32 (33.3) |
| Diabetic neuropathy, n (%) | 12 (12.5) |
| Cause of CKD | 36 (37.5), 15 (15.6), 8 (8.3), 6 (6.2), 22 (22.9) |
| Diabetic nephropathy, GN, ADPKD, NAS, UD; n (%) | |
| GLP-1 RA type: semaglutide, liraglutide, dulaglutide; n (%) | 64 (66.7), 20 (20.8), 12 (12.5) |
| HbA1c (%), median [IQR] | 7.5 [6.5−8.1] |
| Plasma creatinine (mg/dl), median [IQR] | 1.5 [1.1−1.9] |
| eGFR (ml/min/1.72 m2), mean (SD) | 47.3 (18.3) |
| ACR (mg/g), median [IQR] | 128.0 [30.5−567.5] |
| Initiation of GLP-1 RA after KT (months), median [IQR] | 47 [17−104] |
| IS therapy: tacrolimus, mycophenolate mofetil, mycophenolate sodium, everolimus, ciclosporin, prednisone; n (%) | 89 (92.7), 41 (42.7), 45 (46.9), 4 (4.2), 3 (3.1), 90 (93.8) |
ACR: isolated urinary albumin-creatinine ratio; ADPKD: autosomal dominant polycystic kidney disease; BMI: body mass index; CKD: chronic kidney disease; DBP: diastolic blood pressure; DLP: dyslipidaemia; DM: diabetes mellitus; eGFR: estimated glomerular filtration rate; GN: glomerulonephritis; HTN: arterial hypertension; IQR: interquartile range; IS: immunosuppressive; KT: kidney transplant; NAS: nephroangiosclerosis; PTDM; post-transplant diabetes mellitus; SBP: systolic blood pressure; SD: standard deviation; UD: undetermined.
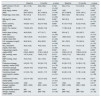
Baseline, six-month and 12-month values of clinical and laboratory test variables compared during follow-up.
| Baseline | 6 months | p-value | Baseline | 12 months | p-value | |
|---|---|---|---|---|---|---|
| eGFR (ml/min/1.73 m2), mean (SD) | 48.1 (17.8) | 49.6 (18.9) | 0.124a (n = 84) | 50.4 (17.4) | 51.8 (18.3) | 0.180a (n = 61) |
| ACR (mg/g), median [IQR] | 100.6 [30.0−525.5] | 81.5 [21.3−342.2] | 0.000b (n = 84) | 108.1 [31.2−246.0] | 61.5 [19.6−193.1] | 0.000b (n = 61) |
| Weight (kg), mean (SD) | 95.1(15.4) | 91.5 (15.7) | 0.000a (n = 84) | 96.0 (15.8) | 92.4 (16.0) | 0.000a (n = 61) |
| BMI (kg/m2), mean (SD) | 35.9 (5.0) | 34.8 (5.2) | 0.000a (n = 84) | 36.3 (5.3) | 34.9 (5.5) | 0.000a (n = 61) |
| HbA1c (mmol/l), median [IQR] | 9.4 [8.2−10.7] | 8.2 [7.4−9.8] | 0.000b (n = 84) | 9.7 [7.9−10.5] | 8.2 [7.3−9.5] | 0.000b (n = 61) |
| HbA1c (%), median [IQR] | 7.5 [6.8−8.3] | 6.8 [6.3−7.8] | 7.7 [6.6−8.4] | 6.8 [6.1−7.7] | ||
| Insulin dose (IU/day), mean (SD) | 49.9 (29.9) | 47.7 (31.7) | 0.048a (n = 65) | 53.9 (4.7) | 52.2 (33.7) | 0.251a (n = 51) |
| Total cholesterol (mg/dl), mean (SD) | 168.7 (40.6) | 157.2 (35.7) | 0.001a (n = 84) | 168.9 (43.0) | 153.3 (32.7) | 0.002a (n = 61) |
| LDL cholesterol (mg/dl), mean (SD) | 89.6 (30.4) | 80.4 (25.5) | 0.002a (n = 84) | 92.1 (33.2) | 75.3 (24.3) | 0.000a (n = 61) |
| HDL cholesterol (mg/dl), mean (SD) | 46.2 (12.8) | 44.5 (12.3) | 0.170a (n = 84) | 44.3 (13.0) | 41.8 (11.8) | 0.380a (n = 61) |
| Triglycerides (mg/dl), median [IQR] | 147.0 [104.0−206.0] | 140.0 [118.0−195.0] | 0.553b (n = 84) | 151.0 [109.0−211.0] | 170.5 [108.5−212.5] | 0.955a (n = 61) |
| SBP (mmHg), mean (SD) | 147.0 (19.4) | 139.5 (17.9) | 0.013a (n = 84) | 145.5 (17.3) | 138.2 (16.0) | 0.004a (n = 61) |
| DBP (mmHg), mean (SD) | 79.4 (10.3) | 79.1 (10.9) | 0.838a (n = 84) | 79.6 (10.4) | 78.7 (9.5) | 0.448a (n = 61) |
| Prednisone dosage (mg/day), median [IQR] | 5.0 [5.0−7.5] | 5.0 [5.0−7.5] | 0.905b (n = 76) | 5.0 [5.0−7.5] | 5.0 [5.0−7.5] | 0.399b (n = 55) |
| Tacrolimus dosage (mg/day), median [IQR] | 3.0 [2.5−5.0] | 3.0 [2.5−4.0] | 0.262b (n = 81) | 3.0 [2.5−5.0] | 3.0 [2.5−5.0] | 0.375b (n = 59) |
| Tacrolimus trough levels (ng/mL), median [IQR] | 6.1 [5.2−7.6] | 6.2 [5.1−7.7] | 0.588b (n = 81) | 6.6 [5.3−7.6] | 5.7 [5.1−6.9] | 0.469b (n = 59) |
| Tacrolimus C/D ratio, median [IQR] | 2.0 [1.4−2.6] | 2.2 [1.3−2.9] | 0.414b (n = 81) | 2.0 [1.6−2.7] | 1.9 [1.1−2.6] | 0.211b (n = 59) |
| Mycophenolate mofetil dosage, (mg/day), median [IQR] | 1000 [625−1,000] | 1000 [500−1,000] | 0.317b (n = 38) | 1000 [500−1,000] | 1000 [500−1,000] | 0.317b (n = 28) |
| Mycophenolate sodium dosage, (mg/day), median [IQR] | 360 [360−720] | 360 [360−720] | 0.414b (n = 39) | 540 [360−720] | 360 [360−720] | 0.785b (n = 32) |
ACR: urinary albumin-creatinine ratio (isolated urine sample); BMI: body mass index; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; HbA1c: glycosylated haemoglobin; IQR: interquartile range; SBP: systolic blood pressure; SD: standard deviation.
Differences between baseline, six-month and 12-month values of the main variables analysed by type of GLP-1 RA.
| Semaglutide | Liraglutide | Dulaglutide | p-value | |
|---|---|---|---|---|
| Starting weight − Weight at 6 months (kg), median [IQR] | 4.0 [1.2−6.9] | 2.7 [−0.8−5.6] | 2.2 [1.2−5.8] | 0.330a |
| (n = 54) | (n = 19) | (n = 11) | ||
| Starting weight − Weight at 12 months (kg), median [IQR] | 3.6 [0.1−7.7] | 3.0 [−0.8−5.0] | 2.3 [1.1−6.3] | 0.827a |
| (n = 39) | (n = 19) | (n = 8) | ||
| BMI start − BMI 6 months (kg/m2), median [IQR] | 1.0 [0.4−2.0] | 1.0 [−0.3−2.1] | 1.3 [0.7−2.2] | 0.778a |
| (n = 54) | (n = 19) | (n = 11) | ||
| BMI start − BMI 12 months (kg/m2), median [IQR] | 1.3 [−0.9−2.5] | 1.5 [0.1−2.8] | 1.2 [0.7−2.6] | 0.779a |
| (n = 39) | (n = 19) | (n = 8) | ||
| eGFR 6 months − eGFR start (ml/min/1.72 m2), median [IQR] | 2.0 [−4.0−5.9] | 3.5 [−3.9−9.0] | −2.5 [−6.7−5.2] | 0.505a |
| (n = 54) | (n = 19) | (n = 11) | ||
| eGFR 12 months − eGFR start (ml/min/1.72 m2), median [IQR] | 0.8 [−4.4−5.7] | 1.5 [−2.5−9.2] | 1.2 [−1.4−7.4] | 0.847a |
| (n = 39) | (n = 19) | (n = 8) | ||
| ACR start − ACR 6 months (mg/g), median [IQR] | 11.3 [−10.8−94.3] | 39.4 [−1.3−78.0] | 53.5 [10.1−146.8] | 0.389a |
| (n = 54) | (n = 19) | (n = 11) | ||
| ACR start − ACR 12 months (mg/g), median [IQR] | 15.6 [−1.0−68.9] | 31.5 [−0.4−120.0] | 60.1 [7.3−129.9] | 0.409a |
| (n = 39) | (n = 19) | (n = 8) | ||
| HbA1c start − HbA1c 6 months (%), median [IQR] | 0.6 [0.1−1.4] | 0.7 [−0.1−1.0] | 1.2 [−0.1−1.5] | 0.556a |
| (n = 54) | (n = 19) | (n = 11) | ||
| HbA1c start – HbA1c 12 months (%), median [IQR] | 0.7 [0.3−1.7] | 0.0 [−0.5−0.8] | 0.7 [0.0−1.3] | 0.058a |
| (n = 39) | (n = 19) | (n = 8) |
ACR: urinary albumin-creatinine ratio in an isolated urine sample; BMI: body mass index; eGFR: estimated glomerular filtration rate; IQR: interquartile range.
Stability in eGFR was observed at six months (p = 0.124) and one year (p = 0.180). We also identified a significant reduction in proteinuria in the ACR at six months (−19.1 mg/g, p = 0.000) and one year (−46.6 mg/g, p = 0.000).
Anthropometric parametersAfter six months of treatment with GLP-1 RA, we found a significant weight reduction (−3.6 kg, p = 0.000), which persisted at one year (−3.6 kg, p = 0.000). BMI was also reduced at six months (−1.1 kg/m2, p = 0.000) and one year (−1.4 kg/m2, p = 0.000).
Blood pressure, glycaemic and lipid controlA significant decrease in systolic blood pressure was observed throughout the follow-up (−7.5 mmHg at six months, p = 0.013; −7.3 mmHg at 12 months, p = 0.004). No significant differences in diastolic blood pressure was observed.
Regarding glycaemic control, glycosylated haemoglobin (HbA1c) values were also lower (−0.7% at six months, p = 0.000; −0.9% at 12 months, p = 0.000). There were no significant differences in steroid or tacrolimus dose that could have had a bearing on these changes.
We also observed a reduction in total cholesterol (−11.5 mg/dl at six months, p = 0.001; −15.6 mg/dl at 12 months, p = 0.002) and low-density lipoprotein cholesterol (LDLc) (−9.2 mg/dl at six months, p = 0.002; −16.8 mg/dl at 12 months, p = 0.000). No differences in triglyceride or high-density lipoprotein cholesterol (HDLc) levels were observed.
Anti-hypertensive drugs, anti-diabetic drugs and statinsChanges in these drugs are shown in Table 4. Insulin dose was reduced after the initiation of the GLP-1 RA (−2.2 IU/day at six months, p = 0.048). No differences were found in the number of subjects treated with oral anti-diabetic drugs during follow-up, except for an increase in the number of subjects receiving SGLT2i at one year (p = 0.031).
Proportion of subjects with different anti-diabetic, anti-hypertensive and statin treatments in the different periods.
| Baselinen = 84 | 6 monthsn = 84 | p-value | Baselinen = 61 | 12 monthsn = 61 | p-value | |
|---|---|---|---|---|---|---|
| Anti-diabetic treatment | ||||||
| Metformin, n (%) | 9 (10.7) | 9 (10.7) | 1.000 | 8 (13.1) | 11 (18.0) | 0.250a |
| SGLT2 inhibitors, n (%) | 3 (3.5) | 5 (5.9) | 0.500 | 2 (3.2) | 7 (11.4) | 0.031a |
| Repaglinide, n (%) | 3 (3.5) | 3 (3.5) | 1.000 | 2 (3.2) | 2 (3.2) | 1.000a |
| Sulfonylurea, n (%) | 1 (1.2) | 1 (1.2) | 1.000 | 0 (0) | 0 (0) | – |
| Anti-hypertensive therapy | ||||||
| ACE inhibitors /ARBs, n (%) | 46 (54.8) | 46 (54.8) | 1.000 | 34 (55.7) | 36 (59.0) | 0.687a |
| Beta-blockers, n (%) | 52 (61.9) | 51 (60.7) | 1.000 | 34 (55.7) | 33 (54.1) | 1.000a |
| Diuretics, n (%) | 23 (27.4) | 24 (28.6) | 1.000 | 16 (26.2) | 17 (27.9) | 1.000a |
| Calcium antagonists, n (%) | 43 (51.2) | 51 (60.7) | 0.125 | 36 (59.0) | 42 (68.9) | 0.031a |
| Alpha blockers, n (%) | 49 (58.3) | 48 (57.1) | 1.000 | 34 (55.7) | 36 (59.0) | 1.000a |
| Statins, n (%) | 67 (79.8) | 68 (81.0) | 1.000 | 47 (77.0) | 50 (82.0) | 0.250a |
The percentage of subjects treated with different anti-hypertensive drugs did not change. We only observed a higher number of subjects treated with calcium antagonists at one year (p = 0.031).
No significant differences were found in the percentage of subjects taking statins and the doses were similar. Only three subjects increased their statin dose during follow-up, and three started statins while they were already on GLP-1 RA treatment.
Adverse effects and safetyIn total, 16 subjects experienced gastrointestinal adverse effects (nausea, vomiting or diarrhea). In five patients, the symptoms improved with the reduction of the GLP-1 RA dose, and the remaining eleven discontinued treatment. There were no changes in the formulation of mycophenolate or the doses owing to gastrointestinal adverse effects. One subject discontinued dulaglutide after eight months of treatment after being diagnosed with pancreatic adenocarcinoma. Three subjects discontinued the drug after prolonged hospital admissions and one subject due to the development of hypoglycaemia. Two deaths were recorded during follow-up: one due to respiratory failure secondary to bilateral COVID-19 pneumonia; and the second subject died after the development of a metastatic lung adenocarcinoma.
Regarding tacrolimus, the doses, trough levels, and the C/D ratio were not significantly altered (Table 2). No episodes of acute rejection were reported, and no dnDSA were detected. Two subjects underwent renal biopsy during the study period because of a progressive deterioration of renal function, with evidence of moderate interstitial fibrosis and tubular atrophy. Only one subject required restarting replacement therapy with hemodialysis during follow-up, but it was not considered to be related to the drug.
DiscussionWe present the results of the largest series reported to date of KT recipients with pre-existing DM or PTDM who received treatment with GLP-1 RAs. In addition, we show data on tacrolimus levels and doses, incidence of acute rejection and potential development of dnDSA during follow-up. These drugs were well tolerated by our subjects, without alterations in immunosuppressive therapy and with improvement in metabolic control and anthropometric parameters. It also observed positive effects on blood pressure control and on parameters of renal function that may offer significant benefits to diabetic KT recipients.
Similar to the efficacy observed in the general diabetic population, in our patients we noticed a significant improvement in glycaemic control, with a decrease in HbA1c levels.4,5 It is important to note that the insulin dose was reduced and there was no change in most anti-diabetic drugs.
GLP-1 RAs can cause gastrointestinal adverse effects that could alter the absorption of immunosuppressive medication and promote renal graft dysfunction. However, several studies point to a potential nephroprotective role of this pharmacological group in other populations, induced by different factors: improvement of hyperglycemia, overweight and insulin resistance, systemic and glomerular hypertension, dyslipidemia, sodium retention, inflammation and renal hypoxia.15,16 In our series, renal function remained stable, in line with that reported in other series of KT recipients.8–10 Therefore, our data appear to confirm the notion that, with appropriate monitoring, GLP-1 RAs are safe drugs regarding graft function.
It was also observed a reduction in body weight and BMI similar to that described in the general population.5,15 This effect has been explained by the delay in gastric emptying and satiety caused by this group of anti-diabetics.4,5
The prevalence of obesity in the KT recipient population is high. This is associated with the development of PTDM, arterial hypertension and dyslipidemia, increasing the risk of death and graft failure.17 In our series we also observed a significant decrease in albuminuria, like in the non-KT population, which is not previously described in other experiences in KT recipients. Albuminuria is also a predictor of mortality and worsening of CKD in KT recipients.18 It has been suggested that body weight influences the development of albuminuria in KT, similarly to that reported in non-KT recipients.18 Our results show a significant decrease in weight, which could explain this decrease in the ACR observed in our subjects. The number of patients treated with SGLT2i at one year was higher, which may also have influenced these findings. Nonetheless, we cannot rule out the direct beneficial effects of GLP-1 RAs on albuminuria, which will need to be the focus of future studies.
Several clinical trials have reported improvement in systolic blood pressure with GLP-1 RAs in non-KT populations.19,20 This may be due to weight loss and the natriuretic and vasodilative effects of these groups of drugs.21,22 However, such findings have not been evaluated or described in the KT population. In our series, we observed an improvement in systolic blood pressure. However, the increase in the number of subjects treated with calcium antagonists at one year of follow-up may have contributed to the improvement in blood pressure control.
In addition, dyslipidemia is a frequent complication after KT and is a risk factor for the development of cardiovascular disease and graft loss.23 Furthermore, most immunosuppressive drugs used in transplantation have a detrimental effect on the lipid profile.24 These alterations have not been adequately analyzed in the previously published series of KT recipients treated with GLP-1 RAs.6–11 In our series, we observed a significant reduction in total cholesterol and LDLc, and it is interesting to note that there were no differences in the number of subjects who received statins or in the dose of those drugs. All this suggests that the benefits derived from GLP-1 RA treatment may help to improve the lipid profile of KT recipients and, consequently, cardiovascular control.
The GLP-1 RAs are eliminated by proteolytic degradation and their metabolism does not involve cytochrome P450 enzymes or transporter-mediated drug interactions.4 Therefore, the probability of interaction with immunosuppressive drugs is low. There is, however, some concern that GLP-1 RAs may affect the absorption of immunosuppressive drugs owing to their role in slowing gastric emptying.4,5 To date, data on tacrolimus doses and levels have been published on a small number of KT recipients treated with GLP-1 RAs.6,8–11 In our study, with a significant number of subjects, trough levels, doses and tacrolimus C/D ratio did not change significantly during follow-up, allowing confidence regarding the safety of immunosuppressive treatment. Furthermore, we observed no episodes of AR, development of dnDSA or an increase in the number of biopsies performed, which also provides safety data about this group of anti-diabetics.
The side effects of GLP-1 RAs are mainly gastrointestinal. Their incidence in clinical trials in the non-KT population ranges between 10% and 50%.21,25 In our series, these adverse effects were not greater than in the general population and they are similar to those reported in other studies in the KT population.6–11 In one of our subjects, GLP-1 RA was discontinued eight months into treatment following a diagnosis of pancreatic adenocarcinoma. In the early years of GLP-1 RA use it was suggested, an increased risk of pancreatitis and pancreatic tumors related to the use of these anti-diabetics.26 However, large clinical trials and other subsequent studies did not confirm this finding accordingly, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) concluded that a causal relationship between these drugs and the development of pancreatitis or pancreatic cancer could not be established.27–31
Our study has several limitations. This was an observational, retrospective, real-life study, without a control group, with the limitations inherent to this design. It would have been interesting to have a comparison arm, but these drugs were prescribed for diabetics with obesity and it was not possible to have a control group with similar characteristics, therefore the results should be considered with caution. Although larger than other series, the number of subjects remains small. No data are available on the adherence to GLP-1 RA treatment, but all subject-reported drug discontinuations were collected. Moreover, conclusions cannot be drawn regarding the influence of these drugs on the dose and levels of everolimus or ciclosporin due to the scarcity of subjects in our series taking these immunosuppressants.
In conclusion, GLP-1 RAs appear to be an option for the management of DM in KT recipients. Our results suggest that they are safe and that they do not appear to alter tacrolimus trough levels or induce episodes of AR or development of dnDSA, with renal function remaining stable. Improvement of metabolic control, weight and blood pressure are of great relevance in this population. For all these reasons, prospective, controlled and randomized studies are warranted to advance our knowledge of the effects of these agents in KT recipients.
FundingThis study received no specific funding from public, private or non-profit organizations.
Conflicts of interestThe authors declare that they have no conflicts of interest.