Chronic kidney disease (CKD) is a serious health problem with an increasing clinical, social and economic impact in advanced stages. Dapagliflozin is a sodium-glucose cotransporter-2 inhibitor that reduces the risk of CKD progression, in addition to provide cardiovascular benefits and reduce all-cause mortality. The aim of this study was to determine the short-term clinical and economic impact of dapagliflozin as an add-on to renin-angiotensin-aldosterone system inhibitors (RAASi) standard therapy for CKD in Spain.
Materials and methodsA cost-offset model was used to compare the costs of clinical events and pharmacological per 100,000 CKD patients in a virtual cohort treated with dapagliflozin added to RAASi standard therapy versus RAASi standard therapy alone. Renal (progression to renal failure and acute kidney injury), cardiovascular (hospitalisation for heart failure [HF]), and all-cause mortality events were assessed. The incidence of clinical events by treatment arm was obtained from the DAPA-CKD study, and costs were obtained from national databases and the literature.
ResultsOver 3 years, treatment with dapagliflozin would reduce progression to renal failure (−33%; 7221 vs. 10,767), hospitalisation for HF (−49%; 2370 vs. 4683) and acute kidney injury (−29%; 4110 vs. 5819). The savings associated with this reduction in events was ;258 million per 100,000 patients, of which 63.4% is due to the avoidance of dialysis for renal failure. Considering the event and pharmacological treatment costs, the total net savings were estimated at ;158 million per 100,000 patients.
ConclusionsDelaying progression of CKD and reducing the incidence of clinical events thanks to the treatment with dapagliflozin could generate savings for the Spanish National Health System, even when pharmacological costs are taken into account.
La enfermedad renal crónica (ERC) es un grave problema de salud cuyo impacto clínico, social y económico se incrementa en estadios avanzados. Dapagliflozina es un inhibidor del cotransportador de sodio-glucosa-2 que reduce el riesgo de progresión de la ERC, además de proporcionar beneficios cardiovasculares y reducir la mortalidad por cualquier causa. El objetivo de este trabajo es determinar el impacto clínico y económico a corto plazo de la adición de dapagliflozina a la terapia estándar con inhibidores del sistema renina angiotensina aldosterona (iSRAA) de la ERC en España.
Materiales y métodosSe empleó un modelo de compensación de costes que comparó los costes de los eventos clínicos y farmacológicos por cada 100.000 pacientes con ERC de una cohorte virtual tratada con dapagliflozina añadida a la terapia estándar con iSRAA frente a la terapia estándar con iSRAA sola. Se evaluaron eventos renales (progresión a fallo renal y lesión renal aguda), cardiovasculares (hospitalización por insuficiencia cardiaca [IC]) y mortalidad por cualquier causa. La incidencia de los eventos clínicos por tratamiento se obtuvo del estudio DAPA-CKD y los costes a partir de bases de datos y literatura nacional.
ResultadosEn 3 años, el tratamiento con dapagliflozina reduciría la progresión a fallo renal (−33%; 7.221 vs. 10.767), hospitalización por IC (−49%; 2.370 vs. 4.683) y lesión renal aguda (−29%; 4.110 vs. 5.819). El ahorro asociado a esta reducción de eventos es de 258 millones de ; por cada 100.000 pacientes, de los cuales el 63,4% corresponde a evitar la diálisis en el fallo renal. Considerando los costes de eventos y los costes farmacológicos del tratamiento, el ahorro total neto se estima en 158 millones de ; por cada 100.000 pacientes.
ConclusionesEl retraso de la progresión de la ERC y la reducción de la aparición de eventos clínicos gracias al tratamiento con dapagliflozina podría generar ahorros para el SNS español, incluso teniendo en cuenta el coste incremental del tratamiento farmacológico.
Chronic kidney disease (CKD) is a serious global public health problem due to its high prevalence and enormous clinical, economic and social impact.1–3
In Spain, this situation is expected to worsen in the near future owing to the ageing of the population and the increased prevalence of other risk factors, such as type 2 diabetes mellitus (T2DM), arterial hypertension and cardiovascular disease (CVD). In a recent study aimed at assessing the clinical and economic burden of CKD in Spain between 2022 and 2027, it was estimated that the prevalence of CKD would increase to 11.7% by 2027, equivalent to 5.68 million patients in Spain, and that approximately two-thirds of these would have silent or undiagnosed progression. Furthermore, it was estimated that the prevalence of patients on renal replacement therapy (RRT) will increase by 14.7%, which would further increase the economic impact associated with CKD on public health spending in Spain, up to ;4.89 billion in 2027.4
CKD progression is associated with the development of complications that have a major impact on people's quality of life, as well as on a social and economic level.5 CKD increases the risk of developing CVD, such as heart failure (HF), and is associated with an increased risk of mortality. Cardiovascular (CV) and all-cause mortality rates are three and 13 times higher, respectively, in patients with category G3-G5 CKD compared to the general population.6,7 Other associated complications include increased risk of acute kidney injury, anaemia, alterations in bone-mineral metabolism and fractures.8 Progression to kidney failure occurs in the most advanced stages of CKD and may require initiation of RRT, resulting in a five- to 10-fold increase in associated costs per patient.9 Therefore, preventing or delaying CKD progression can result in significant quality of life, health and economic benefits.10,11
Dapagliflozin is a sodium-glucose cotransporter-2 inhibitor (SGLT2i) indicated in adults for the treatment of CKD.12 In the Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) study,13 the efficacy and safety of dapagliflozin as an add-on to standard therapy with renin-angiotensin-aldosterone system inhibitors (RAASi) was investigated in 4304 patients with CKD (estimated glomerular filtration rate [eGFR] ≥ 25 and ≤75 ml/min/1.73 m2) and increased albuminuria (urine albumin/creatinine ratio [ACR] between 200 and 5000 mg/g). Dapagliflozin was shown to reduce the risk of progression to kidney failure by 36% (hazard ratio [HR]: 0.64; 95% confidence interval [95% CI]: 0.50−0.82), and a benefit was observed in slowing chronic eGFR decline in treated patients versus placebo, with a mean annual difference of 1.92 ml/min/1.73 m2 (95% CI: 1.61–2.24).14 In addition to the benefit on renal function, dapagliflozin demonstrated a protective effect on HF and mortality compared to placebo, with a relative risk reduction of hospitalisation for HF or death from CV causes of 29% (HR: 0.71; 95% CI: 0.55−0.92; P = .0089) and of the risk of all-cause mortality of 31% (HR: 0.69; 95% CI: 0.53−0.88).14
Cost-offset analyses are a tool that helps those responsible for implementing health strategies and policies to understand the impact of a new intervention compared to other standard of care strategies and to make efficient decisions in their operating environments.15 The objective of this study is to determine the short-term clinical and economic impact associated with the use of dapagliflozin added to standard RAASi therapy in the treatment of CKD compared to standard RAASi therapy alone, from the perspective of the Spanish National Health System (NHS).
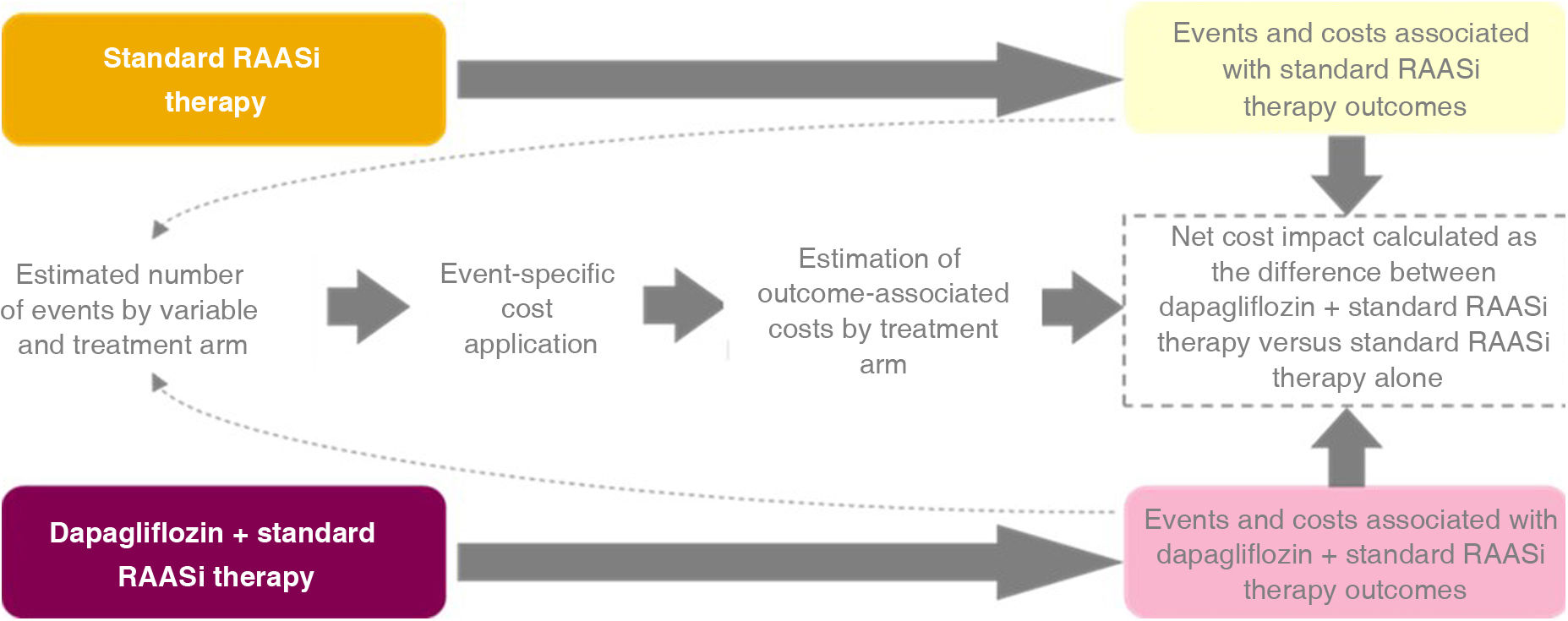
MethodsModel designData on the incidence of events over three years from the DAPA-CKD study were used and these events were transferred to the expected associated costs in the Spanish NHS. For this purpose, we used a cost-offset model (COM), which compares the total healthcare costs of an intervention arm against a comparator that is used as a reference to determine the economic impact of the intervention. Specifically, the costs associated with clinical and pharmacological events were compared in a scenario with dapagliflozin as an intervention versus a scenario with placebo as a comparator, based on the treatment arms of the DAPA-CKD study.13
The conceptual design of the COM is shown in Fig. 1. The intervention arm includes the consequences of treatment with dapagliflozin added to standard RAASi therapy (dapagliflozin + standard RAASi therapy arm), i.e., dapagliflozin added to treatment with angiotensin-converting enzyme inhibitors (ACE inhibitors) or angiotensin II receptor blockers (ARBs). The comparator arm includes the consequences of treatment with standard RAASi therapy alone (standard RAASi therapy arm: ACE inhibitors or ARBs).
For the model, a three-year time horizon was assumed in a virtual cohort of 100,000 patients that reflected the characteristics of the subjects included in the DAPA-CKD study, i.e., patients diagnosed with CKD, with an eGFR ≥ 25 and ≤75 ml/min/1.73 m2, and a urine ACR between 200 and 5000 mg/g.13 The results were also analysed for four specific subpopulations within the population analysed: subjects with previous CKD and T2DM, subjects with CKD without previous T2DM, subjects with CKD and a baseline eGFR ≥ 45 ml/min/1.73 m2, and subjects with CKD and a baseline eGFR < 45 ml/min/1.73 m2.
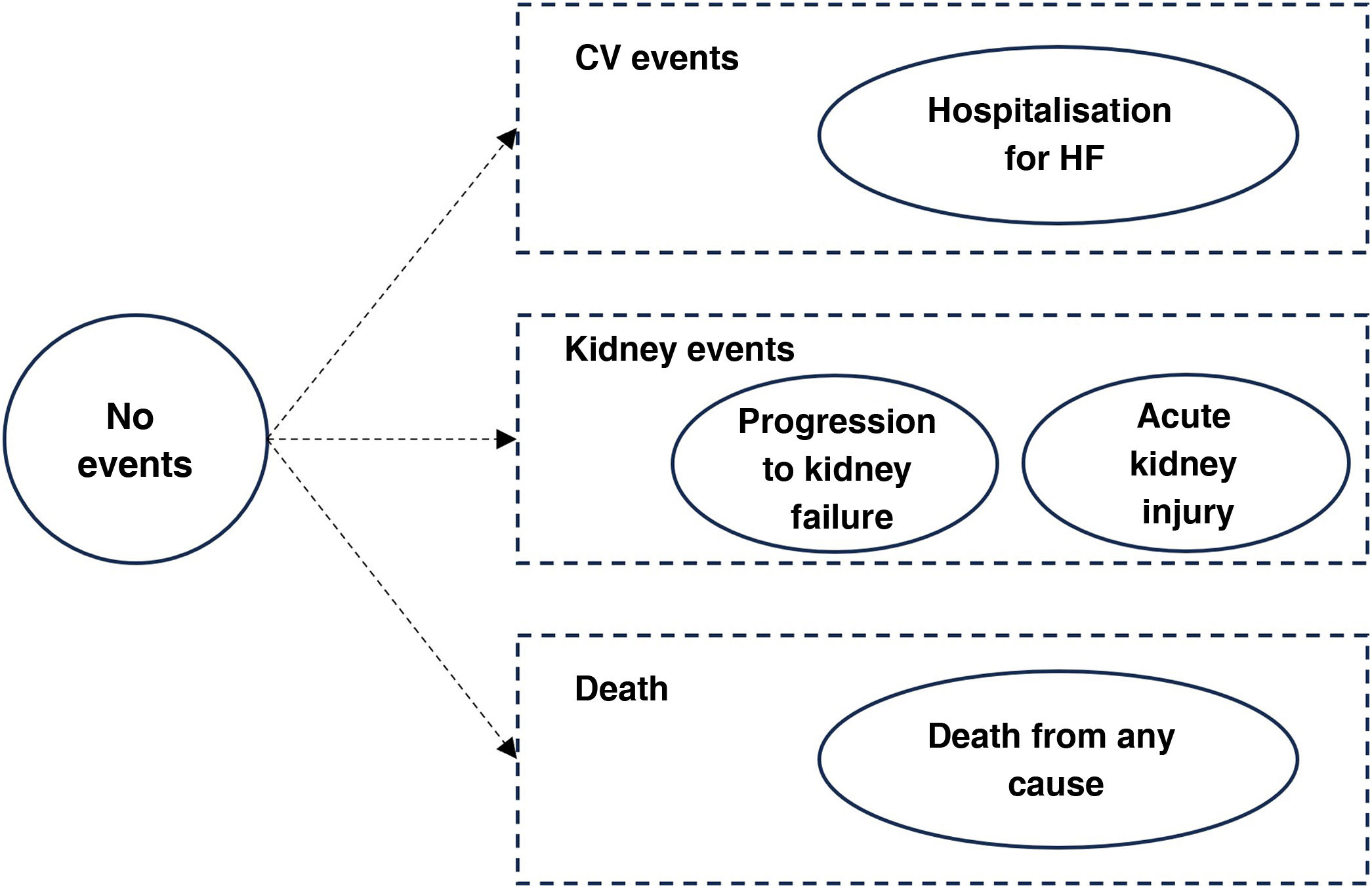
Clinical events and health statusesClinical events were modelled across different health statuses (Fig. 2). The distribution of subjects in each of the health categories was estimated from the event-free survival curves observed in the DAPA-CKD study extrapolated over the time horizon using exponential parametric distributions, assuming that the risks are constant over time. The events progression to kidney failure, acute kidney injury, hospitalisation for HF and death from any cause were included. These events were defined in the same way as in the DAPA-CKD study. A progression to kidney failure event was considered as a decrease in eGFR to below 15 ml/min/1.73 m2 or the need for chronic dialysis or kidney transplant for more than 28 days. The event of severe acute kidney injury was defined as a doubling of serum creatinine compared with the most recent laboratory serum creatinine value.13 The incidence of clinical events by type and treatment arm for all populations analysed was obtained from the DAPA-CKD study.14
Health statuses of the model.
Note: a progression to kidney failure event was defined as a reduction in eGFR below 15 ml/min/1.73 m2 or the need for chronic dialysis or kidney transplant for more than 28 days. Acute kidney injury is defined as a doubling of serum creatinine.
CV: cardiovascular; eGFR: estimated glomerular filtration rate; HF: heart failure.
From the observed events, the number needed to treat (NNT) was calculated, i.e., the average number of subjects who need to be treated with dapagliflozin + standard RAASi therapy rather than with standard RAASi therapy alone to prevent an event.
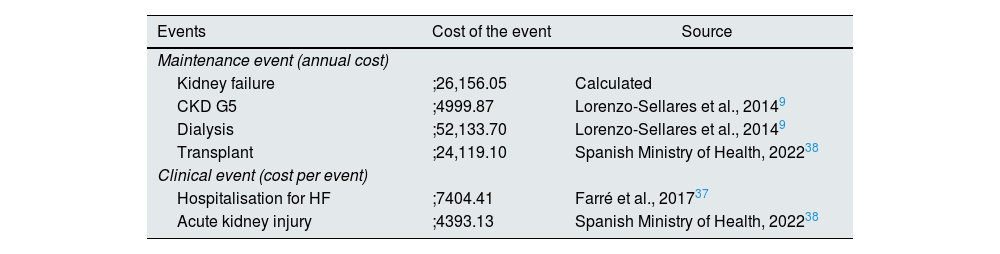
Costs included in the modelCosts associated with clinical events were obtained as part of the global Inside CKD research programme from the published literature (Table 1).16 Clinical event costs were applied just once in the year of event incidence, and maintenance costs were applied from event occurrence until death or the end of the time horizon. The cost associated with mortality was assumed to be zero.
Costs of clinical and maintenance events.
| Events | Cost of the event | Source |
|---|---|---|
| Maintenance event (annual cost) | ||
| Kidney failure | ;26,156.05 | Calculated |
| CKD G5 | ;4999.87 | Lorenzo-Sellares et al., 20149 |
| Dialysis | ;52,133.70 | Lorenzo-Sellares et al., 20149 |
| Transplant | ;24,119.10 | Spanish Ministry of Health, 202238 |
| Clinical event (cost per event) | ||
| Hospitalisation for HF | ;7404.41 | Farré et al., 201737 |
| Acute kidney injury | ;4393.13 | Spanish Ministry of Health, 202238 |
Note: maintenance events are presented as an annual cost and applied in the model as a cost per cycle (daily). A kidney failure event is calculated as a weighted average of the cost of a patient in CKD G5, dialysis and transplant, and the distributions of the population included in the DAPA-CKD study are considered. A single cost is assumed for all types of dialysis, corresponding to the cost of haemodialysis. Clinical events are presented as a cost per event and apply only in the cycle in which they occur. Acute kidney injury is defined as a doubling of serum creatinine. All costs are expressed in ;/2022, updated based on the CPI from the Spanish NIS.
CKD: chronic kidney disease; CPI: consumer price index; HF: heart failure; NIS: National Institute of Statistics.
The cost of the kidney failure maintenance event was adjusted according to the proportion of CKD G5 subjects with and without RRT, with the latter including the cost of dialysis and transplant. It was considered that 53.4% of CKD G5 subjects did not receive RRT, 43.7% received dialysis, and 2.8% had received a transplant, in line with the distribution of patients on RRT in the DAPA-CKD study.14 The cost of peritoneal dialysis was assumed to be equivalent to the cost of haemodialysis.9
Regarding pharmacological costs, the cost of standard RAASi therapy was included for both treatment arms, estimated at ;0.10/day, considering the active substances of the DAPA-CKD study, ACE inhibitors and ARBs, and the distributions of use for each of the active substances based on local literature.14,17 The cost of dapagliflozin treatment was only included in the dapagliflozin + standard RAASi therapy arm, estimated at ;1.07/day. Costs were estimated based on the dosage recommended in the SmPC for all active substances in the treatment of CKD (10 mg/once daily for dapagliflozin).12 The ex-factory price obtained from the database of the Consejo General de Colegios Oficiales de Farmacéuticos [General Council of Official Colleges of Pharmacists]18 was considered, applying the deduction in accordance with Royal Decree Law 8/2010.19
All costs were expressed in euros (;), updated on the basis of the Spanish consumer price index for 2022.20 A discount rate of 3% was used for costs in accordance with the recommendations for carrying out economic evaluations in Spain.21–23
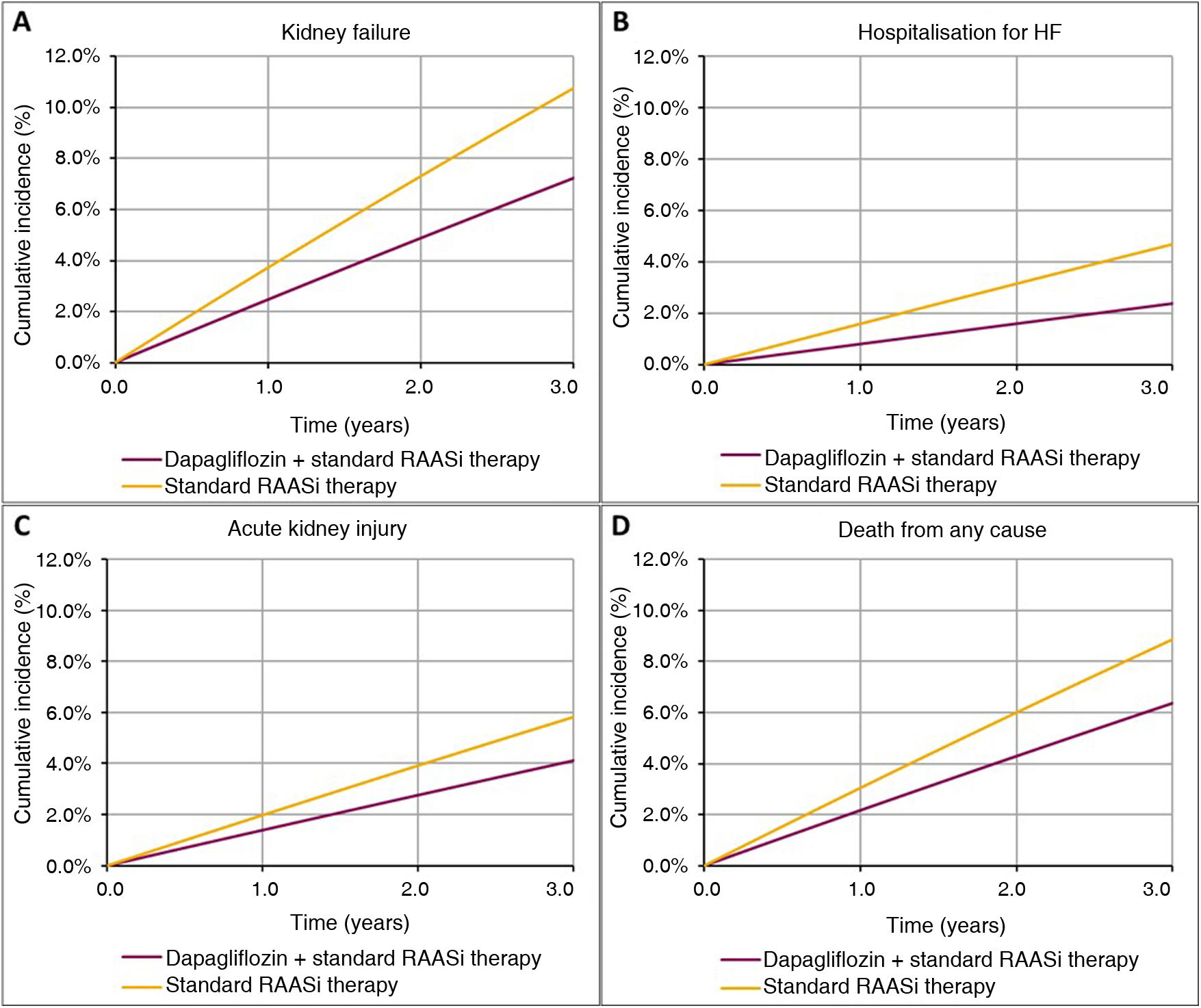
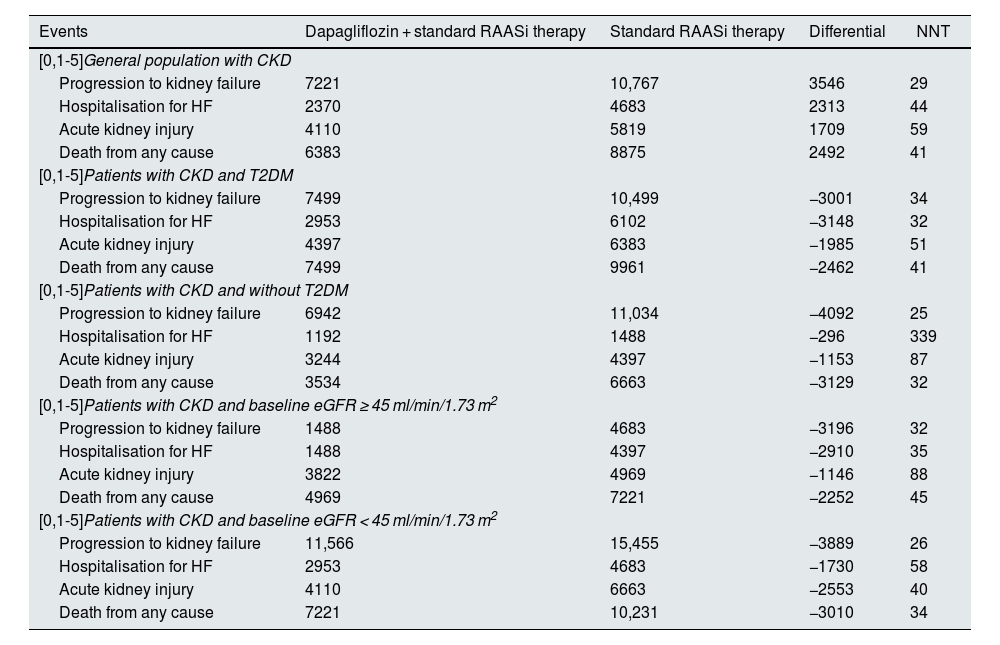
ResultsClinical resultsIn a virtual cohort of 100,000 patients with CKD and baseline characteristics reflecting the inclusion criteria of the DAPA-CKD study (eGFR ≥ 25 and ≤75 ml/min/1.73 m2 and urine ACR 200 to 5000 mg/g), treatment with dapagliflozin would prevent 3546 progression to kidney failure events (1550 dialysis, 102 transplant and 1894 without initiation of RRT), which would represent a 33% reduction in these events, from 10,767 to 7221 over a three-year time horizon. Dapagliflozin would also prevent 2313 hospitalisation events due to HF (−49%; 2370 vs 4683), 1709 acute kidney injury events (−29%; 4110 vs 5819) and 2492 events of death from any cause (−28%; 6383 vs 8875). Table 2 shows events by treatment arm for the population and subpopulations analysed. Fig. 3 shows the cumulative incidence of events per 100,000 subjects in the dapagliflozin + standard RAASi therapy arm and in the standard RAASi therapy arm at 3 years.
Total clinical events per 100,000 subjects/year observed over a 3-year time horizon.
| Events | Dapagliflozin + standard RAASi therapy | Standard RAASi therapy | Differential | NNT |
|---|---|---|---|---|
| [0,1-5]General population with CKD | ||||
| Progression to kidney failure | 7221 | 10,767 | 3546 | 29 |
| Hospitalisation for HF | 2370 | 4683 | 2313 | 44 |
| Acute kidney injury | 4110 | 5819 | 1709 | 59 |
| Death from any cause | 6383 | 8875 | 2492 | 41 |
| [0,1-5]Patients with CKD and T2DM | ||||
| Progression to kidney failure | 7499 | 10,499 | −3001 | 34 |
| Hospitalisation for HF | 2953 | 6102 | −3148 | 32 |
| Acute kidney injury | 4397 | 6383 | −1985 | 51 |
| Death from any cause | 7499 | 9961 | −2462 | 41 |
| [0,1-5]Patients with CKD and without T2DM | ||||
| Progression to kidney failure | 6942 | 11,034 | −4092 | 25 |
| Hospitalisation for HF | 1192 | 1488 | −296 | 339 |
| Acute kidney injury | 3244 | 4397 | −1153 | 87 |
| Death from any cause | 3534 | 6663 | −3129 | 32 |
| [0,1-5]Patients with CKD and baseline eGFR ≥ 45 ml/min/1.73 m2 | ||||
| Progression to kidney failure | 1488 | 4683 | −3196 | 32 |
| Hospitalisation for HF | 1488 | 4397 | −2910 | 35 |
| Acute kidney injury | 3822 | 4969 | −1146 | 88 |
| Death from any cause | 4969 | 7221 | −2252 | 45 |
| [0,1-5]Patients with CKD and baseline eGFR < 45 ml/min/1.73 m2 | ||||
| Progression to kidney failure | 11,566 | 15,455 | −3889 | 26 |
| Hospitalisation for HF | 2953 | 4683 | −1730 | 58 |
| Acute kidney injury | 4110 | 6663 | −2553 | 40 |
| Death from any cause | 7221 | 10,231 | −3010 | 34 |
Note: the general population with CKD refers to patients with baseline characteristics that reflected the inclusion criteria of the DAPA-CKD study (eGFR ≥ 25 and ≤75 ml/min/1.73 m2 and urine ACR 200 to 5000 mg/g).
ACR: albumin/creatinine ratio; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; HF: heart failure; NNT: number of subjects needed to treat to prevent an event; RAASi: renin-angiotensin-aldosterone system inhibitors; T2DM: type 2 diabetes mellitus.
The NNT with dapagliflozin compared with placebo was 29 (95% CI: 27–31) to prevent a kidney failure event, 44 (95% CI: 41−47) to prevent a hospitalisation event due to HF, 59 (95% CI: 53–66) to prevent an acute kidney injury event and 41 (95% CI: 37–45) to prevent an event of death from any cause.
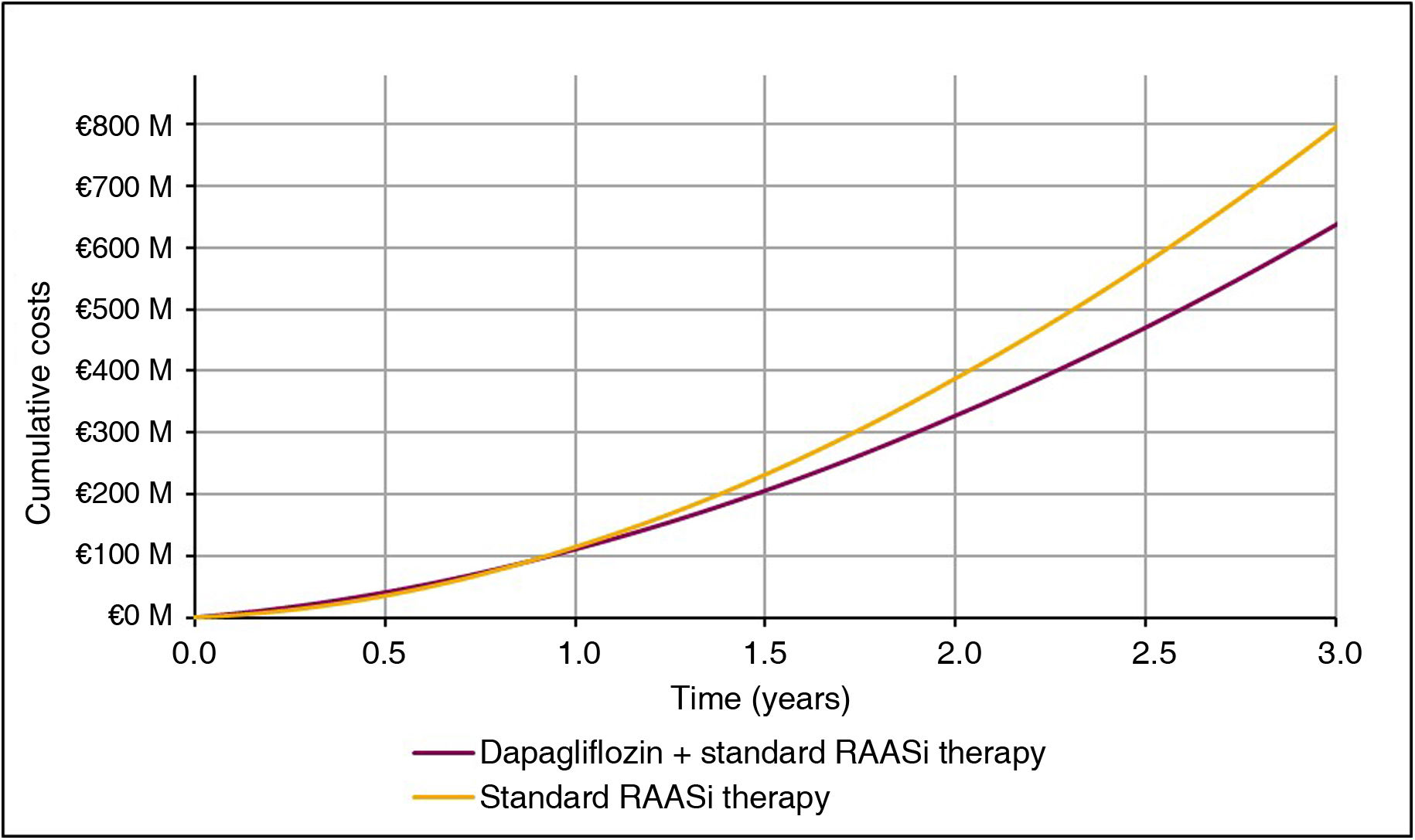
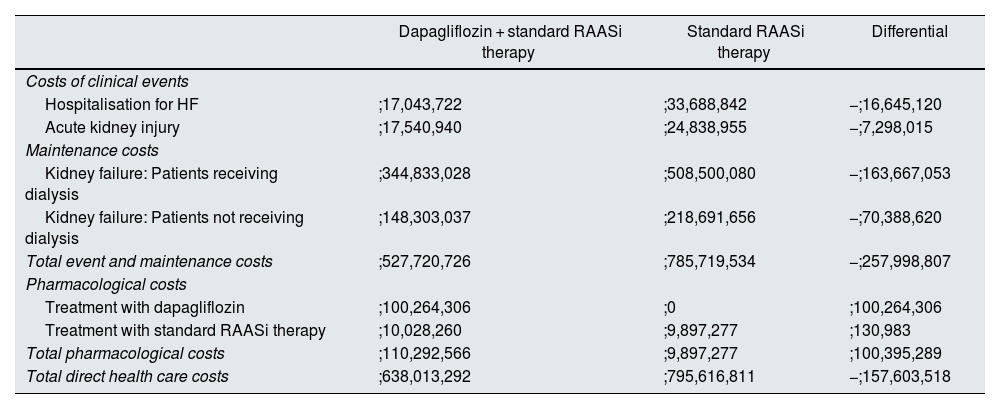
Economic resultsIn line with the clinical results, the difference in cost associated with the reduction in clinical events showed savings for treatment with dapagliflozin + standard RAASi therapy compared to standard RAASi therapy, the most important being the reduction in progression to kidney failure in patients treated with dapagliflozin. Overall, with dapagliflozin treatment, it was estimated that the reduction of incident events and the avoidance of progression to kidney failure, with the consequent chronic maintenance care, reduced healthcare expenditure by ;258 million per 100,000 patients treated over three years, of which 63.4% (;164 million) corresponds to savings from reduced progression to kidney failure in patients requiring dialysis. The economic results for each event are shown in Table 3.
Total cumulative costs over 3 years per 100,000 subjects in the general population with CKD.
| Dapagliflozin + standard RAASi therapy | Standard RAASi therapy | Differential | |
|---|---|---|---|
| Costs of clinical events | |||
| Hospitalisation for HF | ;17,043,722 | ;33,688,842 | −;16,645,120 |
| Acute kidney injury | ;17,540,940 | ;24,838,955 | −;7,298,015 |
| Maintenance costs | |||
| Kidney failure: Patients receiving dialysis | ;344,833,028 | ;508,500,080 | −;163,667,053 |
| Kidney failure: Patients not receiving dialysis | ;148,303,037 | ;218,691,656 | −;70,388,620 |
| Total event and maintenance costs | ;527,720,726 | ;785,719,534 | −;257,998,807 |
| Pharmacological costs | |||
| Treatment with dapagliflozin | ;100,264,306 | ;0 | ;100,264,306 |
| Treatment with standard RAASi therapy | ;10,028,260 | ;9,897,277 | ;130,983 |
| Total pharmacological costs | ;110,292,566 | ;9,897,277 | ;100,395,289 |
| Total direct health care costs | ;638,013,292 | ;795,616,811 | −;157,603,518 |
Note: the general population with CKD refers to patients with baseline characteristics that reflected the inclusion criteria of the DAPA-CKD study (eGFR ≥ 25 and ≤75 ml/min/1.73 m2 and urine ACR 200 to 5000 mg/g). Differential treatment costs with standard RAASi therapy are due to differences in patient mortality in each treatment arm.
ACR: albumin/creatinine ratio; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; HF: heart failure; RAASi: renin-angiotensin-aldosterone system inhibitors.
The pharmacological cost of dapagliflozin + standard RAASi therapy was estimated at ;110 million, and the cost of standard RAASi therapy was estimated at ;10 million per 100,000 subjects treated over three years in the two treatment arms, resulting in an incremental cost of ;100 million per 100,000 subjects treated.
Subtracting the cost of treatment with dapagliflozin from the reduction in health expenditure on events, the net savings from treatment with dapagliflozin + standard RAASi therapy versus standard RAASi therapy would be ;158 million per 100,000 subjects treated over three years (Fig. 4). As such, for each subject treated with dapagliflozin, there would be a saving of ;525.35 per year compared to standard RAASi therapy.
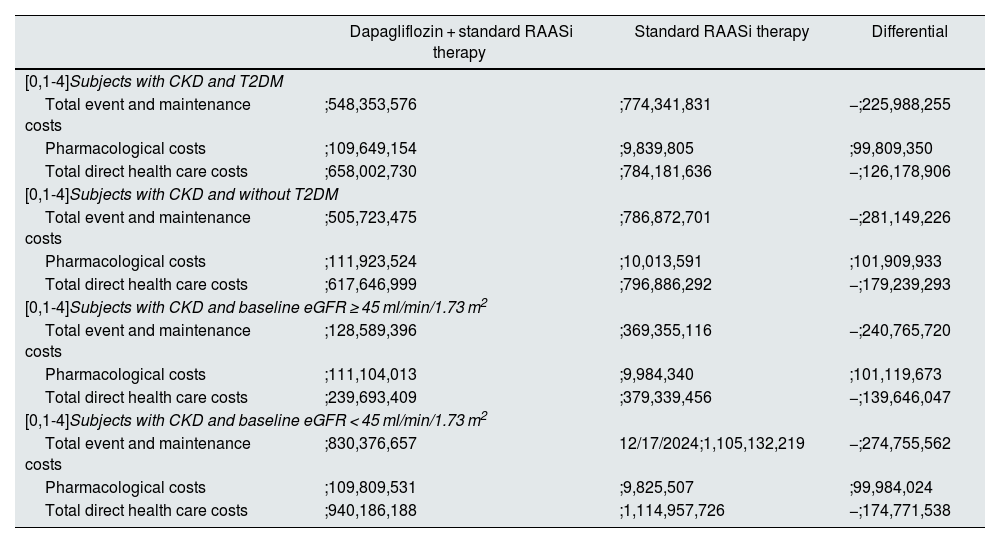
In the subgroup analysis, savings were obtained from the reduction in clinical events in all patient subgroups analysed. Specifically, net savings ranged from ;126 to ;179 million per 100,000 subjects treated over three years, with the greatest savings observed in the subgroup with CKD without T2DM and the subgroup with CKD and a baseline eGFR < 45 ml/min/1.73 m2, mainly due to a greater reduction in progression to kidney failure events (Table 4).
Total cumulative costs over 3 years per 100,000 subjects in the subgroups analysed.
| Dapagliflozin + standard RAASi therapy | Standard RAASi therapy | Differential | |
|---|---|---|---|
| [0,1-4]Subjects with CKD and T2DM | |||
| Total event and maintenance costs | ;548,353,576 | ;774,341,831 | −;225,988,255 |
| Pharmacological costs | ;109,649,154 | ;9,839,805 | ;99,809,350 |
| Total direct health care costs | ;658,002,730 | ;784,181,636 | −;126,178,906 |
| [0,1-4]Subjects with CKD and without T2DM | |||
| Total event and maintenance costs | ;505,723,475 | ;786,872,701 | −;281,149,226 |
| Pharmacological costs | ;111,923,524 | ;10,013,591 | ;101,909,933 |
| Total direct health care costs | ;617,646,999 | ;796,886,292 | −;179,239,293 |
| [0,1-4]Subjects with CKD and baseline eGFR ≥ 45 ml/min/1.73 m2 | |||
| Total event and maintenance costs | ;128,589,396 | ;369,355,116 | −;240,765,720 |
| Pharmacological costs | ;111,104,013 | ;9,984,340 | ;101,119,673 |
| Total direct health care costs | ;239,693,409 | ;379,339,456 | −;139,646,047 |
| [0,1-4]Subjects with CKD and baseline eGFR < 45 ml/min/1.73 m2 | |||
| Total event and maintenance costs | ;830,376,657 | 12/17/2024;1,105,132,219 | −;274,755,562 |
| Pharmacological costs | ;109,809,531 | ;9,825,507 | ;99,984,024 |
| Total direct health care costs | ;940,186,188 | ;1,114,957,726 | −;174,771,538 |
Note: differential treatment costs with standard RAASi therapy are due to differences in subject mortality in each treatment arm.
CKD: chronic kidney disease; T2DM: type 2 diabetes mellitus; eGFR: estimated glomerular filtration rate; RAASi: renin-angiotensin-aldosterone system inhibitors.
The use of SGLT2 inhibitors in patients with CKD is recommended in patients with an eGFR ≥ 20 ml/min/1.73 m2 as a first therapeutic step, together with the use of ACE inhibitors or ARBs, according to the most recent update of the KDIGO international reference guide,24 owing to their demonstrated benefits in reducing disease progression and associated clinical events.14 In this line, this study analysed the clinical and economic impact of short-term CKD associated with treatment with dapagliflozin added to standard RAASi therapy (ACE inhibitors and ARBs) versus standard RAASi therapy in monotherapy in Spain.
It was quantified that treatment with dapagliflozin in subjects diagnosed with CKD would result in a total net saving of ;158 million per 100,000 subjects treated in Spain over three years, including the incremental pharmacological costs of dapagliflozin. This saving is mainly due to the reduction in the number of patients progressing to kidney failure and requiring dialysis, estimated at ;164 million. These net savings were also observed in all patient subpopulations analysed, including patients with a baseline eGFR ≥ 45 ml/min/1.73 m2, where net savings were estimated at ;140 million per 100,000 subjects treated over three years. This would suggest that the introduction of dapagliflozin in early stages, when it is still possible to have a bearing on the course of this disease and reduce progression to kidney failure, is not only necessary from a clinical point of view, but also from an economic perspective.
In this study, the main driver of savings from dapagliflozin treatment is the prevention of events leading to progression to kidney failure, estimated at ;234 million per 100,000 subjects treated in Spain over three years, with a 33% reduction in events compared to standard RAASi therapy. For every 100,000 treated patients with the characteristics of the DAPA-CKD study population and assuming linear progression over time, 1119 patients would initiate RRT (1052 dialysis and 67 transplant) annually in the dapagliflozin plus standard RAASi therapy arm versus 1669 patients annually who would initiate RRT (1569 dialysis and 100 transplant) in the standard RAASi therapy arm. In Spain, the most recent data from the Registro Nacional de Enfermos Renales [National Registry of Kidney Patients] indicate that 7119 patients started RRT in 2022 (6751 dialysis and 368 transplant).25 As a result, considering that, in Spain, between 300,000 and 400,000 subjects with CKD could benefit from treatment with dapagliflozin,26 it is estimated that between 23% and 31% of incident patients on RRT in Spain could be prevented.
In 2023, the first results were published for Inside CKD, the first programme designed to analyse the projected prevalence and burden of CKD in countries around the world and simulate intervention strategies to determine their potential impact on health and economic outcomes on national and global scales.16 In Spain, the projection of the clinical and economic burden of CKD between 2022 and 2027 estimated an increase in the prevalence of CKD in the general population of 5.68 million patients, and a 14.7% increase in the prevalence of RRT in 2027, which would equate to an increase in Spanish public health expenditure of ;4.89 billion in 2027.4 This study highlights the need to intervene in the early stages of the disease to delay progression to kidney failure and demonstrates that it is a unique opportunity to act and be able to slow the progression of the disease through treatment with dapagliflozin.
However, if CKD management continues unchanged, it is expected that up to two-thirds of CKD subjects will have silent progression or will remain undiagnosed, and will therefore not be able to benefit from early-stage treatment.4 Consequently, it is particularly important to follow the recommendations of scientific societies27 and the Spanish Ministry of Health28 that establish the need to determine eGFR and urine ACR in routine tests of subjects at risk of CKD, in order to identify, diagnose and treat early, and reduce CKD progression, as well as the appropriate assignment (in time and form) of CKD diagnostic codes in medical records.27,29,30
Other recent pharmaco-economic evaluations of dapagliflozin therapy in patients with CKD in Spain and other European countries also concluded that treatment with dapagliflozin has the potential to reduce costs. In 2023, McEwan et al. estimated the average economic impact of dapagliflozin treatment + standard RAASi therapy versus standard RAASi therapy in 31 regions around the world, including Spain, based on the same economic model developed with the results of the DAPA-CKD study. However, unlike this study, pharmacological costs were not included. Specifically, it was estimated that treatment with dapagliflozin + standard RAASi therapy versus standard RAASi therapy alone would generate average savings of $264 million per 100,000 subjects over three years, in line with the ;258 million estimated in this evaluation for Spain.31 In 2022, McEwan et al. published a cost-effectiveness analysis of the addition of dapagliflozin to standard RAASi therapy from a payer perspective in three countries: the United Kingdom, Germany and Spain. This study concluded that dapagliflozin therapy is cost-effective versus standard RAASi therapy (ACE inhibitors/ARBs) for the treatment of CKD in the three countries, with an incremental cost-effectiveness ratio of ;9875 per quality-adjusted life year in Spain, considering an efficiency threshold of ;30,000 per quality-adjusted life year.32
There are also studies based on budget impact models that included the costs of clinical events in the evaluation of introducing dapagliflozin for the treatment of CKD. In 2021, Darlington et al. published an analysis of the budgetary impact from a payer perspective in the United Kingdom, including as clinical events CKD progression, progression to kidney failure, hospitalisation for HF, and death. The analysis was conducted on a population of 929,000 potential subjects for treatment with dapagliflozin, and concluded that the total budgetary impact of the introduction of this drug over a three-year time horizon, including pharmacological costs, would result in a saving of £291.3 million compared to standard RAASi therapy.33 In the same vein, in 2023 de Pouvourville et al. published a study of the budgetary impact of the introduction of dapagliflozin from the payer's perspective in France, including pharmacological costs, clinical events (CKD progression, progression to kidney failure, hospitalisation for HF, and death) and adverse events. A total saving of ;650 million was estimated over a five-year time horizon compared to standard RAASi therapy (ACE inhibitors/ARBs), derived from slowed disease progression in subjects treated with dapagliflozin.34
The global perspective of the Inside CKD project suffers from certain limitations in its application to each national setting. The cost estimate for maintaining the kidney failure event was calculated using the proportion of incident patients without RRT, who start dialysis and with initial kidney transplant, based on the results of the DAPA-CKD study.14 It should be noted that the cost of dialysis treatment in Spain varies greatly between different regions and the data used in this study come from one specific region (Canary Islands) and could vary in other settings. It was not taken into account that resources use and patient costs in the first year after transplant are significantly higher than in subsequent years due to the transplant procedure itself.35 This potential overestimation of the cost of transplant may, however, be offset by having assumed, as described in the methods, that 56.3% of CKD G5 subjects do not yet receive dialysis or transplant. Consequently, the weighted cost resulting from maintaining kidney failure is probably lower than expected as subjects will need to start RRT in a short time horizon.
Another possible limitation is that the population included in the model is a defined and closed cohort. The virtual cohort of 100,000 patients in this analysis reflects the baseline characteristics of subjects in the DAPA-CKD study and may not accurately represent the potential population in Spain. However, the population eligible to receive treatment with dapagliflozin in Spain is in line with the inclusion criteria of the DAPA-CKD study,36 owing to which this assumption is not expected to significantly influence the results presented. Comparing the population of the DAPA-CKD study with the data from the IBERICAN national epidemiological study,26 and using conservative criteria, it is estimated that approximately 300,000 to 400,000 subjects would benefit from treatment with dapagliflozin in Spain.
Only events of kidney failure progression, hospitalisation for HF, acute kidney injury and death from any cause were included in this study. Nonetheless, there are other clinical events that could benefit from treatment with dapagliflozin and its improvement could result in greater savings than estimated. Moreover, the unit cost of hospitalisation for HF is highly variable in the literature and depends on the study population (region, comorbidities associated with patients, number of hospitalisations); hence, the savings derived from the reduction in reported hospitalisations could be greater in a population with previous CKD.37,38 Finally, it was not taken into account that treatment with dapagliflozin in patients with CKD would also allow the treatment of other possible comorbidities (e.g., T2DM and HF).39–41 Consequently, the pharmacological costs of standard RAASi therapy could be lower in patients treated with dapagliflozin, and the savings greater than estimated.
ConclusionsTreatment with dapagliflozin has been shown to have key benefits for the management of CKD, such as delaying disease progression and reducing the occurrence of clinical events associated with CKD. This study demonstrates that treatment with dapagliflozin generates savings for the Spanish NHS, even taking into account the incremental cost of drug therapy. The total net savings over three years were estimated to be ;158 million per 100,000 subjects treated with dapagliflozin + standard RAASi therapy, compared with standard RAASi therapy.
FundingInside CKD is funded by AstraZeneca. Inside CKD is a microsimulation data-driven project, so no drugs were supplied or funded. Statistical analysis was funded by AstraZeneca. PharmaLex received funding from AstraZeneca for conducting this study.
AO and JFNG's research is funded by the Community of Madrid in BiomedicineP2022/BMD-7223, CIFRA_COR-CM. Instituto de Salud Carlos III [Carlos III Health Institute] (ISCIII) RICORS programme to RICORS2040 (RD21/0005/0001 and RD21/0005/0013) funded by the European Union – NextGenerationEU, Recovery and Resilience Facility (RRF), ERDF Funds. AO's research is also funded by PREVENTCKD Consortium Project ID: 101101220 Programme: EU4H DG/Agency: HADEA.
The authors express their gratitude to the Global Inside CKD team composed of Salvatore Barone and Claudia Cabrera from AstraZeneca.