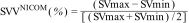Adequate control of patient blood volume in hemodialysis (HD) is essential as a modifiable risk factor for morbidity and mortality. In this study, we propose continuous non-invasive hemodynamic monitoring using bioreactance (Starling SV.Baxter) and real-time characterization of cardiac preload data to aid in the accurate assessment of volume status and improvement of tolerance in HD.
MethodologyObservational and prospective study on the relationship between cardiac preload data and intradialytic hemodynamic instability. Forty-six stable HD patients were recruited. Clinical, analytical, and dialysis data were collected from all participants. The protocol included bioimpedance (BIVA), pre- and post-dialysis echocardiography and tissue Doppler, and monitoring of hemodynamic parameters during the session.
ResultsAccording to the Fall20 definition, 24 patients (51.19%) experienced intradialytic hypotension (IDH). We found no relationship between IDH and analytical, echocardiographic, BIVA parameters, or relative blood volume measurement (BVM) values. Regarding hemodynamic monitoring, indexed systolic volume (ISV) was lower in patients with IDH (38.2 ± 0.9 vs. 39.2 ± 1.9; p < 0.001). Indexed systolic volume variation (ISVV) and heart rate (HR) were higher in the IDH group (14.1 ± 0.7 vs. 13.5 ± 0.7; p < 0.0001), (70.01 ± 2.1 vs. 68.97 ± 1.1; p < 0.0001), respectively. Indexed cardiac output (ICO) and indexed peripheral resistances (IPR) were also lower in the IDH group (2.62 ± 0.09 vs. 2.65 ± 0.13; p < 0.05) and (3201 ± 325 vs. 3432 ± 231; p < 0.05), respectively. Patients who started the session with lower preload (ΔSV after infusion of 250 cc ≥ 10%) more frequently developed IDH (p < 0.001).
ConclusionsNon-invasive hemodynamic monitoring and preload data may constitute a valid tool in managing the volume status of HD patients and preventing IDH.
El adecuado control del volumen sanguíneo del paciente en hemodiálisis (HD) es esencial al ser un factor de riesgo modificable de morbimortalidad. En el presente estudio proponemos la monitorización hemodinámica continua no invasiva por biorreactancia (Starling SV. Baxter) y la caracterización de datos de precarga cardiaca en tiempo real para ayudar a la correcta evaluación de dicho volumen y mejora de la tolerancia en HD.
MetodologíaEstudio observacional, prospectivo sobre la relación existente entre los datos de precarga cardiaca y la inestabilidad hemodinámica intradiálisis. Se reclutaron 46 pacientes estables en HD. Se recogieron datos clínicos, analíticos y de diálisis de todos ellos. El protocolo incluía la realización de una bioimpedancia (BIVA), un ecocardiograma y doppler tisular pre y postdiálisis y la monitorización de parámetros hemodinámicos durante la sesión.
ResultadosConforme a la definición Fall20, presentaron hipotensión intradiálisis (hTAID) 24 pacientes (51,19%). No encontramos relación entre la hTAID y parámetros analíticos, ecocardiográficos, de BIVA ni con valores de medición del volumen de sangre relativo (BVM).
Respecto a la monitorización hemodinámica, el volumen sistólico indexado (VSI) fue menor en los pacientes con hTAID (38,2 ± 0,9 vs. 39,2 ± 1,9; p < 0,001). La variación del volumen sistólico (VVS) y la frecuencia cardiaca (FC) fueron superiores en el grupo con hTAID (14,1 ± 0,7 vs. 13,5 ± 0,7; p < 0,0001), (70,01 ± 2,1 vs. 68,97 ± 1,1; p < 0,0001), respectivamente. El gasto cardiaco indexado (GCI) y resistencias periféricas indexadas (RTPI) también fueron menores en el grupo con hTAID (2,62 ± 0,09 vs. 2,65 ± 0,13; p < 0,05) y (3201 ± 325 vs. 3432 ± 231; p < 0,05), respectivamente.
Los pacientes que comenzaron la sesión con una menor precarga (ΔVS tras infusión de 250 cc ≥10%), desarrollaron con mayor frecuencia hTAID (p < 0,001).
ConclusionesLa monitorización hemodinámica no invasiva y los datos de precarga pueden constituir una herramienta válida en el manejo del estado de volumen de los pacientes en HD y en la prevención de la hTAID.
Chronic kidney disease (CKD), which is generally silent until advanced stages, has become a serious public health problem in recent decades due to the increase in incidence and prevalence, and its high morbidity and mortality.1–4 The main cause of mortality is cardiovascular disease. In patients on renal replacement therapy, it represents 40–50 % of the total, much higher than in the general population, especially among the younger age group.5
Patients on haemodialysis (HD) experience persistent pathophysiological changes that adversely affect different organs. They are the result of numerous systemic stressors, both haemodynamic and non-haemodynamic. They are a combination of cardiocirculatory stress, conditioned by the transition from hypervolaemia to hypovolaemia, hypoxaemia, thermal stress and electrolyte disturbances. These situations occur repeatedly, which globally reduces tissue perfusion and oxygenation.6,7
Acute haemodynamic stress is primarily induced by ultrafiltration, the aim of which is to eliminate excess water accumulated during the interdialysis period. Cyclic changes in blood volume result in chronic cardiac overload and acute volume depletion. Although slight decreases in intravascular volume may go unnoticed, they eventually cause subclinical organic damage that accumulates over years of treatment. In most HD sessions, the vascular refilling rate is not able to compensate for the ultrafiltration rate, leading to a decrease in effective blood volume. Lack of compensation may precipitate intradialytic hypotension (IDH). All of this contributes to the direct relationship between ultrafiltration and hypotension and mortality.8
Conversely, the chronic overload of extracellular volume (vascular and tissue congestion) in HD patients is responsible for hypertension (HTN) and other cardiovascular consequences, also related to morbidity and mortality, including heart failure and acute pulmonary oedema, which are very common in this population.9
In light of the above, adequate volume control is one of the greatest clinical challenges, as it is one of the most important modifiable risk factors for morbidity and mortality associated with HD treatment. The volume status of patients on chronic HD is defined by two components: post-dialysis weight and interdialytic weight gain. Achieving an adequate weight at the end of the sessions is considered a major challenge for nephrologists.10–12
The support on which the determination of fluid balance in HD patients is based is still clinical evaluation; however, volume overload coexisting with a normal physical examination is not uncommon. In an attempt to optimise sensitivity and specificity in estimating dialysis fluid volume, the search for objective assessment tools has intensified.
Different diagnostic methods have been implemented to optimise volume management to avoid chronic overload and haemodynamic instability in HD. These include natriuretic peptides, bioelectrical impedance vector analysis (BIVA) and variation in relative blood volume (BVM, blood volume monitoring) during the session. However, the problem of establishing dry weight and improving tolerance during HD sessions has not yet been resolved. That is why new techniques are required to overcome the shortcomings of traditional methods.13,14
In this study, and to this end, we propose continuous non-invasive haemodynamic monitoring with measurement of cardiac output, stroke volume and peripheral resistance, as well as the characterisation of cardiac preload data in real time. Its relationship with the natriuretic peptide NT-proBNP, with hydration parameters measured by BIVA and with transthoracic echocardiogram is studied.
Material and methodsThis was an observational, prospective study on the relationship between cardiac preload data and haemodynamic instability.
Subjects and data collectionIn total, 46 subjects with CKD on HD for at least three months, clinically stable (with no cardiovascular, cerebrovascular or infectious events in the last month and with no progressive neoplasms), with normally functioning vascular access and adequate dialysis parameters were recruited.
The study protocol was submitted to and accepted by the Complejo Hospitalario Universitario de Toledo [Toledo University Hospital Complex] clinical research committee.
After signing informed consent, subjects were scheduled for the study at the midweek dialysis session (Wednesday or Thursday). They had to come on an empty stomach with the morning medication previously administered at home.
Study protocolUpon arrival at the hospital, the subject had to remain in bed for 30 min and, after recording the pre-dialysis weight, a bioimpedance and transthoracic echocardiogram were performed. They would then connect to their dialysis session. All subjects underwent dialysis with Fresenius 5008 monitors (Fresenius Medical Care, Bad Homburg, Germany) equipped with controlled ultrafiltration and a module for real-time monitoring of relative blood volume (BVM). The dialysis technique used was online haemodiafiltration (online HDF) with a high-flux dialyser (cut-off 78 ml/mmHg) and 1.8 m2 of surface area (Fresenius Polysulfone Helixone Fx80®). The blood flow remained between 350 and 400 ml/min. The dialysis fluid flow was automatically selected by the dialysis monitor and the temperature was set at 36−36.5°C. Clinical parameters were routinely recorded during the session (weight, blood pressure, heart rate [HR], temperature and clinical manifestations, if any).
Haemodynamic monitoringSubjects were haemodynamically monitored from five minutes before the start of the session until 10 min after its completion with the NICOM® bioreactance device (Starling™ SV, Baxter). HR, oxygen saturation (SatO2) and oxygen delivery (DO2I), and cardiac functional parameters, including cardiac output indexed to body surface area and cardiac index (CI), stroke volume (SV), indexed stroke volume (SVi), total peripheral resistance (TPR) and total peripheral resistance indexed to body surface area (TPRi), and thoracic fluid content (TFC) were continuously recorded or calculated. As a surrogate parameter of volume-dependent haemodynamic instability, systolic volume variation (SVV) was determined. It is based on the SVV that is produced with respiratory movements. During spontaneous inspiration, intrathoracic pressure decreases and venous return increases, increasing SV. During expiration the opposite phenomenon occurs. High SVV values indicate that stroke volume varies significantly with changes in preload (volume status), suggesting that the subject is likely to respond positively to fluid administration. Its interpretation in spontaneously breathing subjects should be taken with caution.
The SVV (stroke volume variation) determined with the NICOM® device was averaged every 60 seconds. Within this time interval, the maximum and minimum SV values were selected for each 15-beat subsegment. The final maximum and minimum SV values were determined from several segmental maximum and minimum SV values in each 60-second time window. The final SVVNICOM was then calculated as:
Two infusion manoeuvres of a 240-cc bolus of fluid were performed online. The first immediately before the start of dialysis, and the second at the end of the session. Based on the Frank-Starling law, a bolus infusion was used to assess cardiac preload on the basis of the increase experienced by SVi after volume infusion (Fig. 1).15
Frank-Starling law. The increase in stroke volume of a study subject after the infusion of 250 cc of volume (online fluid) in a pre- and post-dialysis is presented as an example.
At the end of the session, a new bioimpedance test and an echocardiogram were performed, thus completing the study protocol.
The haemodynamic parameters and their normal values are shown in Table 1.
Haemodynamic parameters and normal values.
| Haemodynamic parameter | Formula | Normal value |
|---|---|---|
| Stroke volume (SV) | CO/HR × 1,000 | 60−100 ml/beat |
| Stroke volume index (SVi) | SV/Body surface area | 33−47 ml/beat/m2 |
| Cardiac output (CO) | HR × SV/1,000 | 4.0−8.0 l/min |
| Cardiac index (CI) | CO/Body surface area | 2.5−4.0 l/min/m2 |
| Total peripheral resistances (TPR) | 80 × (MBP)/CO | 800−1,200 dynes • s/cm5 |
| Total peripheral resistance index (TPRi) | 80 × (MBP)/CI | 1,970−2,390 dynes • s/cm5/m2 |
| Mean blood pressure (MBP) | (SBP + [2 × DBP])/3 | 70−105 mmHg |
| Δ Stroke volume index (ΔSVi) | SVi change after 250-cc serum infusion online | ≥10% volume responder<10% non-responder to volume |
Continuous variables are expressed as mean ± SD or median and interquartile range (IQR) depending on the nature of the variables. Categorical variables are expressed as percentages. As tests of independence, Student's t-test was used, and with three or more categories means were compared by analysis of variance (ANOVA). Before applying a parametric test, Levene's homogeneity of variance test was applied. In non-parametric tests, the Mann-Whitney U test was used for two categories, and the Kruskal-Wallis test for three or more groups. The Wilcoxon signed-rank test was used to compare the different temporal states of our variables. All statistical tests are applied with a 95% confidence level. To perform the analyses, the statistical software IBM SPSS® version 29.0 was used.
ResultsBaseline description of the cohortThe analysis includes 46 subjects with CKD 5D from the hospital's HD unit. The subjects' time on replacement therapy was 7.74 months (IQR: 3.35). The mean age was 63.4 years (IQR: 22.4). Overall, 69.6% (32) were male and 47.8% (22 patients) were diabetic. The Charlson comorbidity index of the study population was 7 (IQR: 4). The baseline data of the study population are expressed in detail in Table 2.
General subject characteristics: laboratory values and dialysis characteristics.
| General characteristics | |
| Male, N (%) | 32 (69.6%) |
| Age, (years) | 63.4 (IQR: 22.4) |
| Time on dialysis, (months) | 7.74 (IQR 3.35) |
| Diabetes mellitus, N (%) | 22 (47.83%) |
| Ischaemic heart disease, N (%) | 12 (26.89%) |
| Vascular disease, N (%) | 8 (17.39%) |
| Heart failure, N (%) | 11 (23.9%) |
| Charlson index | 7 (IQR: 4) |
| ®-blockers, N (%) | 18 (39%) |
| Laboratory values | |
| Haemoglobin, (g/dl) | 11.8 ± 1.5 |
| Haematocrit, (%) | 35.7 ± 4.5 |
| Creatinine, (mg/dl) | 7.7 ± 2.7 |
| Na+, mEq/l | 139 ± 3.5 |
| K+, (mEq/l) | 5.2 ± 0.7 |
| Bicarbonate, (mEq/l) | 23.9 ± 3.3 |
| Albumin, (g/dl) | 3.8 ± 0.4 |
| Ca++, (mg/dl) | 9.2 ± 0.7 |
| P, (mg/dl) | 4.6 ± 1.5 |
| NT-proBNP, (pg/mL) | 8,008 ± 11,031 |
| Troponin I, (ng/mL) | 0.03 ± 0.05 |
| Dialysis characteristics | |
| HD time, (min) | 230 ± 15.3 |
| Qb, (ml/min) | 380 ± 35.5 |
| Kt (L), convective dose, (l) | 54.5 ± 8.4/25.4 ± 3.5 |
| ID weight gain, (l) | 2.5 ± 1.1 |
| UF rate, (ml/h) | 0.65 ± 0.21 |
| Total UF, (l) | 1.6 ± 0.84 |
All patients were dialysed on Fresenius 5008 monitors, using the online HDF technique with a Helixone dialyser. The characteristics of the dialysis sessions and the clearances of the technique are shown in Table 2.
Intradialytic hypotensionDifferent criteria were used to define intradialytic hypotension (IDH). The incidence of hypotension varied according to the different classifications. In our study we used Fall20. In total, 24 patients (51.187%) presented with IDH (Table 3). Data on systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean blood pressure (MBP), as well as data on dialysis dose, interdialytic weight gain and ultrafiltration rates are presented in Table 4. No differences were found in the incidence of IDH when comparing patients with or without diabetes.
Pre- and post-dialysis blood pressure and subjects with intradialytic arterial hypotension based on the Fall20 definition.
| pre-HD | post-HD | p-value | |
|---|---|---|---|
| SBP (mmHg) | 147.21 ± 22.1 | 145.28 ± 27 | NS |
| DBP (mmHg) | 79.21 ± 13.1 | 79 ± 14 | NS |
| MBP (mmHg) | 103 ± 12.6 | 102.2 ± 15.9 | NS |
| Intradialytic hypotension (Fall20) | 24 (52.17%) | ||
Pre- and post-dialysis blood pressure and dialysis characteristics of subjects with intradialytic arterial hypotension based on the Fall20 definition.
| Hypotension [Fall20] | No hypotension | p-value | |
|---|---|---|---|
| Pre-HD SBP, (mmHg) | 157.3 ± 22.5 | 138 ± 17.4 | 0.002 |
| Pre-HD DBP, (mmHg) | 81.2 ± 14 | 77.4 ± 12.2 | 0.32 |
| Pre-HD MBP, (mmHg) | 108.4 ± 12.3 | 97.6 ± 10.6 | 0.004 |
| Post-HD SBP, (mmHg) | 145.2 ± 27.7 | 145.3 ± 26.9 | 0.99 |
| Post-HD DBP, (mmHg) | 77.4 ± 14.3 | 80.5 ± 13.9 | NS |
| Post-HD MBP, (mmHg) | 101.6 ± 16.5 | 102.9 ± 15.7 | NS |
| Session time, (min) | 235.8 ± 10 | 227 ± 17.18 | 0.06 |
| Qb, (ml/min) | 378 ± 42 | 381.9 ± 31.4 | 0.7 |
| KT dose, (l) | 54.8 ± 9.4 | 54.3 ± 7.8 | 0.8 |
| Convective dose (l) | 26.1 ± 3.9 | 24.9 ± 3.1 | 0.2 |
| Inter-HD weight gain, (l) | 2.5 ± 1.3 | 2.6 ± 0.8 | 0.8 |
| UF rate, (ml/h) | 0.62 ± 0.24 | 0.67 ± 0.19 | 0.47 |
| UF rate, (ml/kg/h) | 5.9 ± 3.1 | 6.3 ± 2.5 | 0.62 |
| Total UF (l) | 1.57 ± 0.92 | 1.75 ± 0.76 | 0.43 |
The results in bold denote statistically significant values.
We found no relationship between haemoglobin (Hb), calcium, albumin, troponin I and NTproBNP with the presence or absence of IDH or diabetes. NTproBNP was associated with ischaemic heart disease (15,937 ± 14,224 vs. 5,450 ± 8,593; p = 0.007), peripheral vascular disease (18,540 ± 14,705 vs. 5,456 ± 8,388; p = 0.002) and heart failure (17,142 ± 16,646 vs. 5,439 ± 7.381; p = 0.004). Troponin I was associated with ischaemic heart disease (0.072 ± 0.07 vs. 0.021 ± 0.019; p = 0.001) and peripheral vascular disease (0.091 ± 0.076 vs. 0.021 ± 0.019; p < 0.001).
BIVA parametersData on overhydration (OH), total body water (TBW), extracellular water and the extracellular/intracellular (E/I) ratio, pre- and post-dialysis were comparable in the groups with and without IDH.
BVM parametersHourly RSV values were also unable to discriminate the population that developed hypotension during dialysis.
Echocardiographic parametersAll patients had a transthoracic echocardiogram prior to and at the end of the dialysis session. Left ventricular end-diastolic volume (LVEDD) decreased from pre- to post-dialysis (46.92 ± 7.2 vs. 45.3 ± 7.3 mm; p = 0.0015). The left ventricular end-systolic volume (LVESD) (31.3 ± 6.2 vs. 30.1 ± 7 mm; p = 0.011), right ventricular (RV) size (38.6 ± 6.3 vs. 37.2 ± 6.1 mm; p = 0.03), the left atrium (LA) (69 ± 27.7 vs. 61.4 ± 25.2 mm; p = 0.009) and left ventricular (LV) mass (205.9 ± 73.7 vs. 186.8 ± 50.8; p = 0.016), behaved similarly. The tricuspid annular plane systolic excursion (TAPSE) (21.62 ± 4.9 vs. 20.15 ± 4.6; p = 0.0007) and the E/A wave ratio (1.02 ± 0.4 vs. 0.84 ± 0.3; p < 0.0001) were also modified. There were no differences in left ventricular ejection fraction (LVEF), E´ wave or the E/e´ ratio.
No differences were found in echocardiographic parameters when comparing subjects who became hypotensive versus those who did not. The complete data are shown in Table 5.
Pre- and post-dialysis echocardiographic parameters of subjects with and without intradialytic arterial hypotension.
| Echocardiographic parameters | Hypotension [Fall20] | No hypotension | p-value |
|---|---|---|---|
| Pre-HD LVEDD | 47.2 ± 8.6 | 46.1 ± 5.5 | 0.6 |
| Post-HD LVEDD | 46 ± 8.3 | 44.3 ± 6.06 | 0.42 |
| Pre-HD LVESD | 32.1 ± 7.4 | 30.2 ± 4.9 | 0.34 |
| Post-HD LVESD | 31 ± 8.1 | 29.3 ± 5.5 | 0.46 |
| Pre-HD LVEF | 59.8 ± 12.6 | 61.47 ± 10.14 | 0.62 |
| Post-HD LVEF | 59.7 ± 13 | 62.6 ± 10.6 | 0.41 |
| RV (pre-HD) | 37.8 ± 7.1 | 39.1 ± 5.2 | 0.51 |
| RV (post-HD) | 36 ± 5.8 | 38.5 ± 6.1 | 0.17 |
| Pre-HD LA (cm) | 63.5 ± 28.9 | 67 ± 28.7 | 0.73 |
| Post-HD LA (cm) | 59.5 ± 22.7 | 62.1 ± 22.7 | 0.76 |
| Pre-HD TAPSE | 21.3 ± 5.3 | 22.2 ± 4.3 | 0.55 |
| Post-HD TAPSE | 19.9 ± 5.2 | 20.4 ± 3.9 | 0.71 |
| Pre-HD S wave | 11.13 ± 3.2 | 12.3 ± 2.2 | 0.17 |
| Post-HD S wave | 11.2 ± 2.8 | 11.8 ± 3.3 | 0.57 |
| Pre-HD E/A | 0.9 ± 0.4 | 1.06 ± 0.41 | 0.19 |
| Post-HD E/A | 0.78 ± 0.28 | 0.93 ± 0.37 | 0.14 |
| Pre-HD E/e’ | 12.4 ± 6 | 12.6 ± 7.7 | 0.93 |
| Post-HD E/e’ | 11 ± 3.3 | 12.1 ± 8.9 | 0.6 |
| Pre-HD LV mass | 202.15 ± 73.1 | 206 ± 77.2 | 0.8 |
| Post-HD LV mass | 191.4 ± 60 | 180.02 ± 32.2 | 0.46 |
Overall, indexed stroke volume (SVi) was higher in patients without IDH (39.2 ± 1.9 vs. 38.2 ± 0.9; p < 0.001). Throughout dialysis in the group of patients without IDH, SVi fell in the first hour (41 ± 2.1 vs. 38.1 ± 0.6; p < 0.0001), recovering in the following hours (38.3 ± 0.7 and 39 ± 1.9; p < 0.001). In the IDH group, the SVi showed a fall that lasted until the last hour of dialysis (38.6 ± 1 [1st h] vs. 38.2 ± 0.9 [2nd h] vs. 37.7 ± 0.9 [3rd h] vs. 38.5 ± 1.2 [4th h]; p < 0.001) (Fig. 2).
Comparison of haemodynamic parameters between groups: (a) Indexed cardiac output; (b) Indexed stroke volume; (c) Variation in stroke volume; (d) DO2I: O2 delivery to tissues; (e) O2saturation; (f) Heart rate; (g) Thoracic fluid content; (h) Systolic, diastolic and mean blood pressure; and (i) Peripheral resistances.
Studying the SVV over time, as a parameter of haemodynamic stability, we observed that the SVV was significantly higher in the group with IDH (14.08 ± 0.69 vs. 13.53 ± 0.74; p < 0.0001).
The overall HR was higher in patients who became hypotensive (70.01 ± 2.1 vs. 68.97 ± 1.1; p < 0.001). In both groups, the frequency increased throughout dialysis, with a greater increase seen in the group with haemodynamic instability.
Indexed cardiac output (COi) was slightly lower in the group that developed arterial hypotension (2.62 ± 0.09 vs. 2.65 ± 0.13; p < 0.05). Throughout the session, cardiac output remained highly stable, despite the fall in SVi, thanks to the compensation produced by the increase in HR.
Indexed peripheral resistances (IPR) were lower overall in the IDH group (3,201 ± 325 vs. 3,432 ± 231; p < 0.05). Resistances did not change in the group in which blood pressure (BP) remained stable, while in the group with IDH, peripheral resistances fell from the beginning of dialysis (3,856.4 ± 22.6 [1st h] vs. 3,441.1 ± 228.7 [2nd h] vs. 2,973.1 ± 127.7 [3rd h] vs. 3,028.2 ± 179.9 [4th h]; p = 0.033). IPRs were not different when comparing subjects with or without diabetes.
O2 saturation decreased in the first hour of dialysis in both groups, then improved until the end of the session. No differences were found between the groups with and without IDH. Oxygen delivery to the tissues (DO2I) remained stable during the dialysis session with no differences between the groups with and without IDH.
Finally, thoracic fluid content (TFC) was determined. An increase in TFC was observed in the first hour of dialysis, with a decrease in the following hours, more pronounced and sustained in the group with IDH.
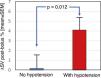
Cardiac preload parametersAs cardiac preload parameters, the increase in stroke volume after the infusion of a 250-cc bolus of solution online, comparable to the leg raising manoeuvre, were used. This manoeuvre was performed prior to and at the end of dialysis. The increase in SV in pre-dialysis was 2.15 ± 7.2% vs. 13.1 ± 8.7% post-dialysis; p < 0.001 (Fig. 3). Patients who developed hypotension during the pre-dialysis session had a greater increase in post-bolus SV compared to those who maintained stable blood pressure (0.17 ± 7.72 vs. 4.12 ± 6.19; p = 0.012) (Fig. 4). Post-dialysis, there was no difference between both groups (11.9 ± 9.15 vs. 14.36 ± 8.28; p = 0.68). Patients who started and finished the session with higher depletion data (ΔSV ≥10% after the bolus), that is, with a lower preload, were those who presented with greater haemodynamic intolerance during the HD session (χ2 (1,N = 46) = 20.45; p < 0.001) and (χ2 (1,N = 46) = 5.23; p = 0.02), respectively.
Adequate volume control in HD patients is one of the greatest clinical challenges for nephrologists, as it is one of the fundamental modifiable risk factors for morbidity and mortality associated with HD treatment. Inadequate volume management can lead to haemodynamic instability and affect patient health. Understanding the associated factors and their consequences remains an area of interest and debate in the scientific literature. In recent decades, different diagnostic methods have been implemented to optimise volume management in dialysis patients and avoid chronic overload and IDH during HD sessions, including applications based on artificial intelligence.16,17
In this study, we propose non-invasive monitoring and characterisation of cardiac preload data with the aim of preventing the deleterious effects of both volume overload and instability in HD.
We included 46 subjects from a hospital HD unit, comprehensively addressing the complexity of the interaction between HD and cardiovascular response. The characteristics of the cohort, the existence of IDH and its relationship with HD laboratory and echocardiographic parameters are described. This comprehensive approach sheds light on the complex relationship between HD and cardiovascular response, providing valuable information for the clinical management of these subjects.
In total, 51% of subjects presented with IDH (Fall20). The predictive power of non-fatal cardiovascular events and of overall mortality for IDH, with asymptomatic definitions, magnifies the importance of hypotensive episodes regardless of whether or not they are accompanied by associated symptoms.18 Flythe et al.19 have demonstrated the predictive power of mortality of the Nadir90 definition in patients with a pre-dialysis SBP of around 130 mmHg; in our study, the pre-dialysis SBP was higher than 140 mmHg, owing to which we decided to use the Fall20 definition with asymptomatic SBP drops, but higher than 20 mmHg in the short time span of the HD session.
On the basis of this definition, IDH did not correlate with any of the laboratory parameters studied. Neither anaemia levels nor values of cardiovascular disease-related peptides (NTproBNP and troponin I) were able to predict or discriminate those subjects who would subsequently present with a drop in SBP during the HD session. However, and as expected, both peptides were related to the existence of cardiovascular disease in its different forms. Some groups have described a direct relationship between NTproBNP values, interdialytic weight gain (IDWG) and ultrafiltration (UF), provided that this was greater than 0.6% IDWG/h.20 In our case, the IDWG and UF were lower than those of this study, which could explain the absence of findings in this regard. In contrast, Curtis et al.21 postulate that elevated NTproBNP levels in incident patients may identify subjects with better dialysis tolerance and tolerance of high UF rates. However, the results of our study lead us to think, in line with other groups,22 that the predictive value of the peptides studied is fundamentally related to structural pathology and not to acute functional phenomena: in our case subclinical.
Regarding echocardiographic parameters, LVEDD, LVESD, RV volume and LA volume decreased between pre-dialysis, with greater volume overload and preload, and the post-dialysis period. However, it was not discriminatory between those who developed IDH and those who did not. Since left ventricular mass was calculated using the Devereux formula23 and this includes the volumes of the cardiac cavities, this also decreased from the pre-dialysis to the post-dialysis period. Once again, echocardiographic parameters were not sensitive to differentiate the group of patients with and without IDH.
Diastolic dysfunction in patients with nephropathy is gaining increasing interest. Its prevalence is estimated to be between 48 and 73% in dialysis patients.24 The difficulty in detecting it lies in its definition. For its definition, both the E/A ratio (<1) and the E/e’ wave ratio (>10) have been used. The diagnosis would include not only tissue Doppler echocardiographic parameters, but also clinical parameters. Currently, interest is focused on the detection of early and asymptomatic abnormalities.
The E and A waves correspond to measurements of the passive and active filling velocities of the LV, respectively. These velocities can be altered if the preload is very high; hence, the E/A ratio is not sufficient for diagnosis as the sole criterion. For this reason, it must be combined with other measures.
In our research, the Doppler study of the mitral annulus shows a normal diastolic function in terms of the relationship of the E/A waves, which clearly worsens in the post-dialysis echocardiogram. This deterioration in the dialysis session could once again be attributed to the decrease in preload volumes.
Another of the criteria used to assess diastolic dysfunction is the relationship between the E/e’ waves. In this case, although no significant difference was found between the pre- and post-dialysis period, the ratio in both cases was less than 10, which has been linked to an increased risk of cardiovascular mortality.25
Subjects with diastolic dysfunction are more susceptible to fluctuations in ventricular volume and hypovolaemia, which, even if mild, can cause IDH. In our study, we found no differences between the population with and without IDH.
RV systolic function, as measured by TAPSE, also deteriorates in relation to the fall in preload after ultrafiltration of the dialysis subject. Again, no differences were found between subjects who became hypotensive and those who did not.
The haemodynamic changes between groups in the absence of clinical symptoms were probably not sufficiently determinant of intradialytic cardiac stunning, and could explain this lack of differences. The fact that intravascular volume depletion, measured by cardiac chamber shrinkage and other echocardiographic parameters, does not differ between the two populations, supports the multifactorial and complex nature of IDH and the need to continue exploring other tools that help us predict such events.26
In our study, haemodynamic monitoring was performed with a non-invasive bioreactance device. The parameters described above were recorded minute by minute throughout HD.
Overall, SVi was lower in patients with IDH. In both groups, SVi fell within the first hour, recovering in the second half of dialysis only in subjects who remained stable without IDH.
There were also differences in SVV, which was higher in subjects who presented with a fall in BP. As described in the literature, SVV is an independent predictor for developing IDH,27 since subjects with a higher SVV have an insufficient compensatory response to intravascular volume changes.
The COi was higher in the non-hypotensive group, but in both the IDH and stable BP groups, it remained stable throughout dialysis. Despite the decrease in SVi that we observed during HD, the progressive increase in HR allowed the COi to remain stable.
In the IDH group, IPRs decreased from the start of dialysis, and were lower than in the stable BP group. This fall in resistance is probably one of the most important components in the development of IDH in our study. The distribution of subjects with or without diabetes was equal between the groups with and without IDH. Although dysautonomia is common in diabetic subjects, there were no differences between IPRs in subjects with and without the disease.
Other parameters monitored throughout the session are related to "oxygenation" and thoracic fluids. O2 saturation and DO2I decrease in the first hour of dialysis and then recover. The TFC behaves in the opposite way, increasing at the beginning of the HD and then decreasing until the end of the session. We relate these results to the phenomena of bioincompatibility in dialysis, mediated by complement activation, the sequestration of neutrophils in the pulmonary vessels and other factors, such as the reduction of respiratory stimulus due to the elimination of CO2 and the rapid increase in plasma bicarbonate.
An essential part of the study was the assessment of cardiac preload both prior to and at the end of HD. Having continuous monitoring allowed us to measure the increase in stroke volume after infusing a 240-cc bolus of fluid online. SV response to this intravascular volume overload served as a method for assessing preload. Classically, two groups of subjects are considered: those with low preload are called "volume responders" if they have IDH (ΔSV ≥10%); and those with high preload or "non-responders to volume" (ΔSV <10%) which includes patients with intravascular volume overload. In our study, subjects who became hypotensive had a lower predialysis preload; i.e., the equivalent of a situation of lower intravascular volume overload, without this being discriminated by the values of OH, TBW and extracellular water (ECW) in the BIVA. This probably needs be related to the inability of the BIVA to independently assess intravascular volume.28
We compared the groups according to their volume response (ΔSV). In both pre- and post-dialysis, the subjects considered “volume responders”, irrespective of their current BP, were those who had IDH.
One of the most important aspects in the routine clinical practice of dialysis patients is the estimation of dry weight, closely related to the haemodynamic tolerance of HD subjects. An adequate adjustment of dry weight remains the greatest challenge for nephrologists, given its poor correlation with physical examination. This has led to a constant search for tools in order to adequately estimate this characteristic. A unified concept defines it as "the lowest tolerated post-dialysis weight achieved through gradual change in post-dialysis weight in which there are minimal signs or symptoms of hypovolaemia or hypervolaemia".29 Achieving dry weight by simplistically using blood pressure can put the patient at risk, and its appearance cannot be considered as a marker of having reached or exceeded dry weight.
For this reason, finding new methods that allow us to evaluate the volume of these patients could prevent IDH, including asymptomatic IDH, minimising tissue damage.
Our study found no relationship with tools such as BIVA, which is widely used for estimating dry weight. Nonetheless, real-time haemodynamic monitoring has allowed us to measure preload parameters that can identify those patients who are at risk of hypotension.
An interesting aspect that also arises from preload monitoring and the ability to discriminate patients as volume responders or non-responders to volume, based on their (ΔSV), is its involvement when making decisions regarding the clinical management of these patients. When hypotension occurs at a time during the dialysis session where their ΔSV <10%, the therapeutic strategy should be different from infusing volume or decreasing the UF rate, since, far from improving BP, it could lead to volume overload, which is also deleterious in these patients. Based on the data obtained in our study, the objective of the treatment would be to improve the peripheral resistance of this group of patients. Not all cases of hypotension in dialysis are volume dependent, as there is also a percentage that would be dependent on amines or vasoconstrictor drugs.30
The most important limitation of this study is the small sample size. It results in lower statistical power. From a clinical point of view, it is worth noting the use of UF rates lower than those usually prescribed. In the design of this study, the objective was to assess subclinical changes in patients with IDH, whether or not induced by ultrafiltration. With higher rates of UF and the onset of hypotension with associated symptoms, more evident echocardiographic or haemodynamic changes would probably have been revealed.
Further studies with a larger number of subjects are warranted to validate haemodynamic monitoring as a useful tool for volume management and to prevent subclinical hypotensive events.
In conclusion, in our study, the classical methods used to assess the volume status of dialysis patients, such as natriuretic peptides, BIVA and transthoracic echocardiogram, were not sensitive in detecting intradialytic SBP falls (Fall20). The detection of these intradialytic haemodynamic changes is of great importance owing to their association with cardiovascular morbidity and mortality. We have shown that non-invasive haemodynamic monitoring and cardiac preload data can be valid tools in the management of volume status by showing greater sensitivity to prevent subclinical falls in BP, and to administer pathophysiologically appropriate therapies and improve the long-term prognosis of HD patients.
FundingThis study has received no specific funding from public, private or non-profit organisations.