El tacrolimus (Tac) es un inmunosupresor ampliamente usado para prevenir el rechazo en el trasplante renal. Los pacientes reciben una dosis inicial estándar y se miden los niveles sanguíneos, con ajuste de la dosis hasta alcanzar una concentración dentro del rango aceptado. Existe una gran variabilidad interindividual en las dosis necesarias para alcanzar ese nivel diana en sangre, y muchos pacientes requieren varias modificaciones de la dosis hasta alcanzarlo. Uno de los principales determinantes de estas diferencias es un polimorfismo del gen CYP3A5 que determina que alrededor del 80 % de los caucásicos sean metabolizadores lentos y requieran dosis menores que los metabolizadores rápidos. Se ha propuesto que los pacientes trasplantados reciban dosis iniciales de Tac con base en el genotipo CYP3A5. Para que este procedimiento fuese aceptado por los clínicos, deberían demostrarse sus ventajas frente al procedimiento actual, más allá de un menor tiempo para alcanzar la dosis óptima. Por ejemplo, menor tasa de nefrotoxicidad y rechazo o menor coste por necesitar, entre otros, menos modificaciones de la dosis de Tac y menos terapia de inducción con anticuerpos.
Tacrolimus (FK-506) is an immunosuppressant widely used to prevent kidney transplant rejection. Patients receive an initial standard dose and tacrolimus levels are measured in blood. If necessary, the dose is adjusted to reach a blood concentration within the accepted range. There is great interindividual variability in the dose required to achieve the target blood level, and many patients require multiple modifications of the dose to reach the range. One of the main determinants of these differences is a CYP3A5 gene polymorphism that determines that about 80% of Caucasians are poor metabolisers and require lower doses compared to the extensive metabolisers. It has been proposed that transplanted patients could receive an initial Tacrolimus dose based on the CYP3A5 genotype. This could reduce the time to achieve the optimal blood level, reducing the number of dose modifications. However, to be accepted by clinicians and translated to the clinical practice this adapted dose procedure should give additional advantages such as a significant reduction of the rates of nephrotoxicity and rejection, or a lower cost due to less dose modifications of Tacrolimus and less antibody induction therapy.
INTRODUCTION
The goal of immunosuppressive therapy is to prevent graft rejection. For this purpose drugs acting by different routes on the alloimmune response are administered, to achieve the highest degree of immunosuppression while minimising toxicity and other adverse effects, especially the development of tumours and infections.1 The recommended immunosuppressive therapy is an initial ‘induction’ therapy with antibodies (monoclonal or polyclonal), directed at preventing early rejection, and reduced doses of other immunosuppressives, especially calcineurin inhibitors, and maintenance immunosuppression with three drugs: Calcineurin inhibitors, such as tacrolimus or cyclosporine A, an inhibitor of purine synthesis (mycophenolate mofetil [MMF]) and steroids.2 Other immunosuppressive agents such as mTOR (mammalian rapamycin receptor) inhibitors (sirolimus and everolimus), although not usually used from the beginning, make it possible to use different combinations of immunosuppressants over time.
For most drugs each patient receives an initial dose based on variables such as weight and age. If drug blood levels can be measured, the dose may be adjusted to a blood value within an acceptable range. With regards to immunosuppressive drugs, a dose that is too low could cause organ rejection, and a dose that is too high could cause toxicity. Gene variations (mutations and polymorphisms) that encode proteins involved in drug metabolism may condition blood concentration of the pharmacologically active ingredient. Proteins encoded by these pharmacogenetically relevant genes may act at intestinal absorption level (thus affecting the amount of drug absorbed into the bloodstream) or liver, resulting in inactive or therapeutically active molecules. Finally, they can modify the drug or its metabolites to facilitate their elimination. For most genes involved in these processes there are polymorphisms that determine more or less active forms of proteins, so that, based on the corresponding genotypes, it would be possible to include each patient in a group with a better/worse therapeutic response. In this review we analyse the pharmacogenetics of tacrolimus, discussing the pros and cons of choosing a dose based on the patient's genotype.
TACROLIMUS: MECHANISM OF ACTION, PHARMACOKINETICS AND PHARMACODYNAMICS
Tacrolimus (FK-506) is an inhibitor of calcineurin, a phosphoprotein that promotes transcription of genes involved in growth and differentiation of CD4+ T lymphocytes. When tacrolimus binds to its intracellular target, there is inhibition of transcription of genes such as interleukin-2.3This immunosuppressant is characterised by its pharmacokinetic variability (interindividual) and its reduced therapeutic window.4 Therefore, continuous dose monitoring is required during the first weeks after transplantation in order to achieve adequate blood levels to prevent rejection (too low) or nephrotoxicity (too high).5,6 Although rapidly absorbed (in the intestine), its oral bioavailability is very low: of the total dose the patient receives only 25% would reach the blood stream. Maximum blood concentration is achieved within one to three hours after administration. Administration, either in two divided doses (Prograf ®, Astellas ®) or a single daily dose in the case of an extended release form (Advagraf ®) should be administered within 24 hours after transplantation, although depending on the donor’s features there may be differences between the centres, both in administration regimes and doses, as also combinations with other immunosuppressants. In the case of renal transplantation, the Symphony study recommends an initial oral dose of tacrolimus in the range of 0.10-0.30 mg/kg/day, depending on donor and recipient characteristics and associated immunosuppressive medication.7
In the intestine, tacrolimus is a substrate for P-glycoprotein (Pgp) (also known as MDR-1 -multidrug resistance 1-), encoded by the ABCB1gene.8-10This protein is found in the membrane of enterocytes and regulates the passage of substances from the interior of the cell into the extracellular space based on an adenosine triphosphate (ATP) mechanism. Once in the blood stream, tacrolimus reaches the liver where it is metabolised by CYP3A family cytochrome P450 reductases, primarily CYP3A5 and CYP3A4.11 Finally, its metabolites are excreted in urine and bile. The narrow therapeutic margin and large pharmacokinetic and pharmacodynamic variability between individuals make it necessary to measure tacrolimus blood levels and those of its metabolites to adjust the dose to achieve an appropriate circulating concentration.12 There are various measuring methods based on the use of antibodies (ELISA) or mass spectrometry. Monitoring trough levels of tacrolimus (C0) is critical during the first days after transplant and is performed immediately before each administration. In the case of renal transplant, the Symphony study recommends that C0 levels in whole blood values should be between 5 and 20ng/ml during the first weeks after transplant, and between 3 and 12ng/ml when the patient changes to maintenance therapy.7 Monitoring and dose adjustment should be performed when immunosuppressive regimen changes are introduced, or changes in the dosage form, or after administration of other drugs that could interfere with absorption and/or metabolism (such as clopidogrel and simvastatin). Dose modifications are also necessary when nephrotoxicity is suspected.13,14
PHARMACOGENETICS OF TACROLIMUS
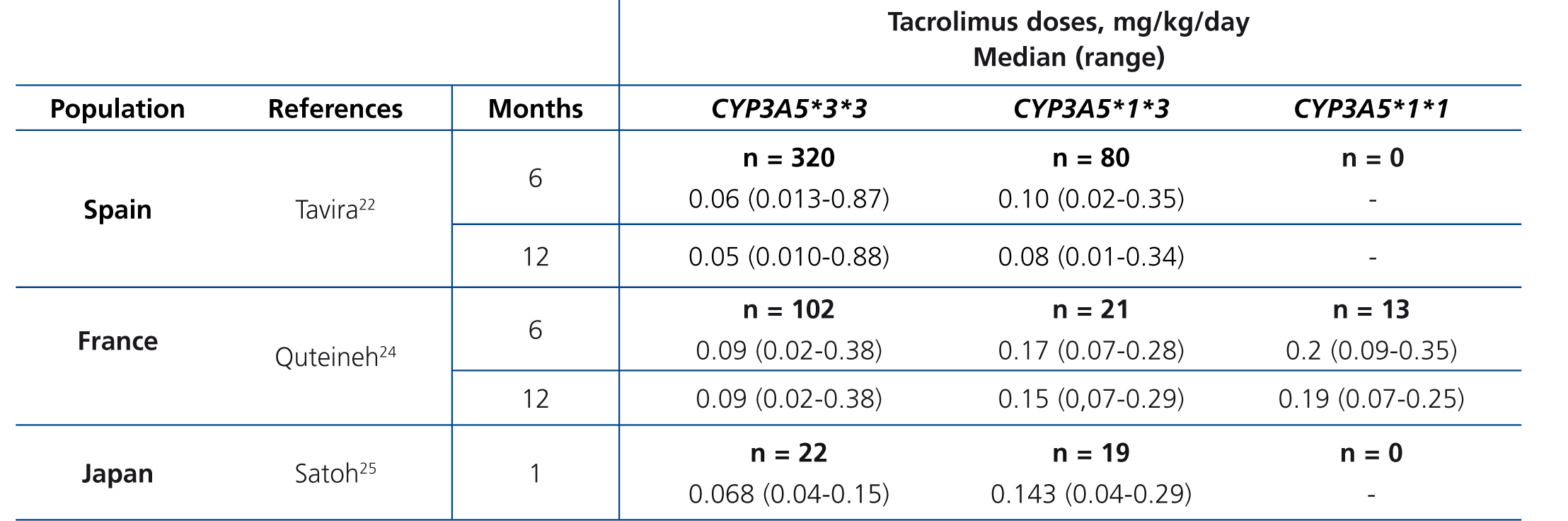
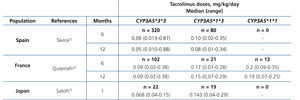
The main determinant of interindividual variability in the dose of tacrolimus is the activity of cytochrome P450-3A5, encoded by CYP3A5 gene. Of all the polymorphisms of this gene, a change in a single nucleotide (SNP), known as CYP3A5*3 (SNP rs776746), is the main regulator of the optimum dose.15-19 This variant is in intron 3 of the CYP3A5 gene and affects pre-mRNA processing, so that there is not a perfect splicing between exons 3 and 4. The resulting mRNA will have an abnormal sequence, which is unstable and will be eliminated by the cell, so that it fails to synthesise protein.20 21 Consequently, carriers of two copies (homozygous) of the CYP3A5*3 allele do not have the protein (non-expressers), in contrast to carriers of at least one copy of the normal allele (designated CYP3A5*1).22-26 In summary, CYP3A5*3 would make it possible to classify patients according to phenotype as “slow metabolisers” (homozygous CYP3A5*3*3), “intermediate metabolisers” or “fast metabolisers” (heterozygous CYP3A5*1*3 and homozygous CYP3A5*1*1, respectively). The latter would require higher doses to achieve target levels of tacrolimus (Table 1).24-26 Other gene variants might be involved in the metabolism of tacrolimus, although the results obtained to date are not as conclusive as those seen with CYP3A5*3.27-30 Differences in allele frequencies of CYP3A5 gene between ethnic groups could explain the increased dose requirement in African Americans: while about 80 % of Caucasians are slow metabolisers (homozygous for the CYP3A5*3 allele), most black subjects are homozygous CYP3A5 *1*1 (fast metabolisers ).31.32These genetic differences could also explain the increased risk of rejection and nephrotoxicity among Afro-Americans.33
About 40 % of tacrolimus administered to a patient is metabolised by P450-3A4. Variants of gene CYP3A4 have been found that could affect the activity of this cytochrome and, therefore, basal drug levels/doses. In the case of tacrolimus CYP3A4*1B polymorphism (SNP rs2740574) in the promoter region of the gene (-392 A> G), perhaps resulting in higher protein levels, has been associated with a requirement for greater drug doses.22,34,35 In addition, some variants have recently been described that could affect tacrolimus metabolism, such as CYP3A4*22 polymorphism.36,37 However, other studies do not support a significant effect of this variant of CYP3A4 on doses.38,39 When assessing these discrepancies it is necessary to be aware that most carriers of CYP3A4*22 allele in turn are homozygous for CYP3A5*3 allele, which makes it difficult to quantify its effect on fast metabolisers for CYP3A5.39
ABCB1 gene encodes PgP, a protein that is expressed in many cell types and tissues and regulates intestinal absorption of many drugs. A relationship has been described between C3435T polymorphism (SNP rs1045642) within exon 26 of the gene and intestinal expression of this glycoprotein. Thus, this (and other polymorphisms) could condition dosage requirements for several drugs.40, 41 In the case of tacrolimus several studies found a significant relationship between dose requirement and C3435T genotype polymorphism.17, 28.42 However, this effect has not been confirmed by others, nor does it affect the dosage level in our patients.17,22,28,42-44 The expression of PgP in renal cells could condition the amount of tacrolimus reaching their interior and, therefore, toxicity levels. In this respect, a relationship has been described between donor C3435T genotype and the risk of nephrotoxicity.45 Although this study would open up a new pharmacogenetic pathway in renal transplant, it is based on a small number of patients, and should be confirmed in other populations.
CLINICAL APPLICATION OF TACROLIMUS PHARMACOGENETICS
The initial dose of tacrolimus was determined by variables such as patient age, weight or race.7,46,47 Some authors have proposed algorithms based on different variables to calculate the initial dose, including CYP3A5 genotype, which should be determined before transplant. In theory, the inclusion of genotype could reduce the number of dose adjustments and the time necessary to reach the desired blood level. For several drugs, the choice of a dose based on genotype is already a recommendation from the Food and Drug Administration and other agencies. In the case of tacrolimus, for the medical community to accept pharmacogenetics as a prescription criterion, patient genotype should provide significant advantages with regards to current procedures: for example, reducing the percentage of rejection or other adverse events, fewer modification of post-transplant doses; savings by reducing pharmaceutical expenditure or hospital stay, etc. However, so far no studies have clearly demonstrated the advantages of giving a variable initial dose by genotype compared to the current method of a predetermined dose.
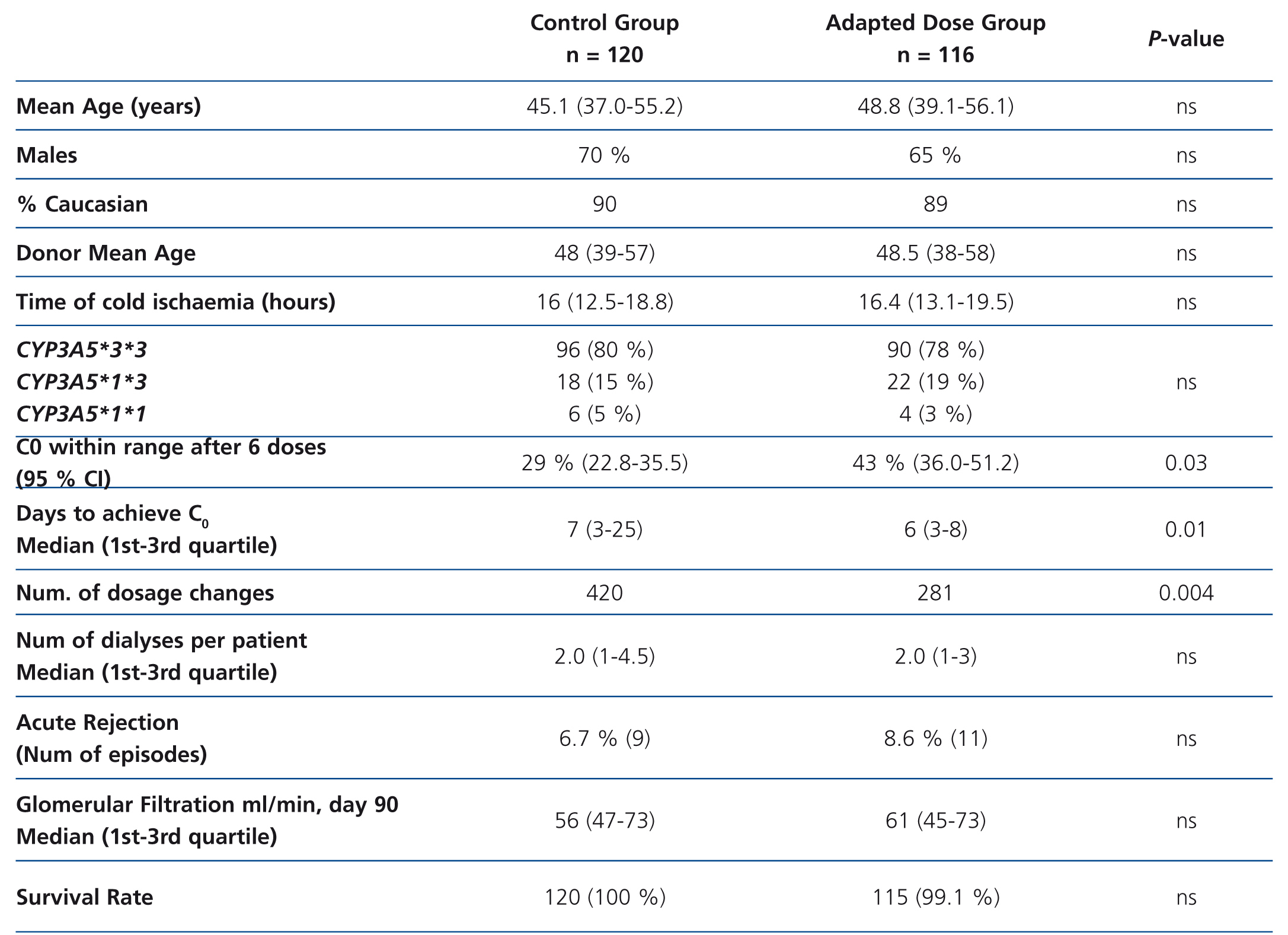
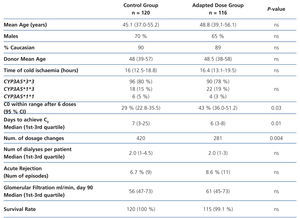
To demonstrate these advantages, a group of patients would have to be treated using an initial dose based on genotype to compare the results with a group of similar characteristics (age, sex, immunosuppressive scheme) to which tacrolimus is administered following traditional guidelines. This approach has been used in a study performed on 236 renal transplant patients divided into two groups: 120 patients (control group) received 0.2 mg/kg/day and 116 patients (adapted dose group) received a dose according to CYP3A5 genotype: 0.15 mg/kg/day in the case of slow metabolisers (homozygous for CYP3A5*3*3, n = 90) and 0.30 mg/kg/day in the case of fast metabolisers (CYP3A5*1 carriers, n = 26).48 However, patients began to receive tacrolimus as from post-transplant day 7 (not from the first day), and were kept under low induction therapy with basiliximab (Simulect®; Novartis, Basel, Switzerland) or anti-thymocyte globulin (Thymoglobulin®; Genzyme, Cambridge, MA) during that week. The two groups were compared based on the percentage of cases with a C0 value in the 10-15ng/l range after six oral doses of tacrolimus, time to achieve that range, number of dose modifications to achieve that range, number dialysis sessions to organ function, incidence of acute rejection (biopsy-proven ), loss of organ and death.48 The study concluded that after three days of treatment with tacrolimus there were a greater percentage of patients achieving target value C0 (43.2% vs. 29.1%, P=.03) in the adapted dose group, and these patients also required fewer dose modifications. However, the incidence of acute rejection or renal function values were similar between groups (Table 2). The sample size would be sufficient to achieve a statistical power of 80%.
Since measuring levels and adjusting the daily dose would make it possible for most patients to achieve target blood levels within the first two weeks after transplant, nephrologists may see little use in dose by genotype in the absence of other advantages. Since the study performed by Thervet et al. included patients treated with induction therapy and high doses of MMF, it is not surprising that there were no differences in the rate of acute rejection and other clinical variables. Therefore, it would be necessary to evaluate the role of dosage according to genotype in patients without induction therapy and administering tacrolimus immediately after transplant, instead of waiting for a week.49
CONCLUSIONS
While the role of CYP3A5 genotype as a determinant of the dose of tacrolimus to administer is indisputable, the usefulness of giving an initial dose based on genotype may depend on greater benefits than the mere reduction in the number of dose modifications. More trials are necessary to demonstrate that administration of adapted doses reduces nephrotoxicity and rejection, or saves money due to lower requirements for induction therapy or shortens hospitalisation times.
KEY CONCEPTS
ACKNOWLEDGEMENTS
Study financed by the Renal Research Network of the Carlos III Institute of Health (RD12/0021/0012; RD12/0021/0018).
Table 1. Daily dose of tacrolimus (median and range) according to CYP3A5 genotype in three series of kidney transplants
Table 2. Summary of the study performed by Thervet et al.48