knowledge of a disease could be equated with the construction of a building, so that the first brick is required for the second, and thus onwards on to get as close as possible to the end. In the case of tubulopathies, that ‘construction’ was very difficult because it required an accomplished understanding of renal tubular physiology, which greatly increased when molecular biology techniques were developed. Some steps of the history of knowledge of familial hypomagnesaemia with hypercalciuria and nephrocalcinosis (FHHNC) have been added and reported in Spain. This is the story.
DESCRIPTION OF THE DISEASE AND SOME NAMES GIVEN WHEN IT WAS NOT YET KNOWN IF IT WAS AN INDIVIDUAL ENTITY. THE FIRST CASES PUBLISHED IN SPAIN
Although no eye anomalies are mentioned, it is possible that three papers at the end of the sixties and in the seventies described the first published cases of FHHNC.1-3 In 1969, Alfrey and Jenkins wrote a clinical history of a 16-year-old boy who had suffered from the condition since he was five years of age.1 Magnesaemia was very low (0.8mg/dl), and magnesium clearance was elevated for the level of serum magnesium (10ml/min). The young man had polyuria and radiological nephrocalcinosis demonstrated by a concentration defect resistant to pitressin (260 mOsm/l), which seemed to contribute to the existing hypokalaemia (2.5-3.2mEq/l), given the secondary hyperaldosteronism. There was not hypercalciuria, but the patient showed data compatible with renal failure (79ml/min). In 1972, Michelis et al. published a report based on data from two siblings with nephrocalcinosis, vasopressin-resistant polyuria and hypomagnesaemia (1.1-1.2mEq/L) secondary to tubular loss, one of whom suffered convulsions.2 Blood bicarbonate levels in the first of the brothers was low (19mEq/l) with a slightly elevated chloraemia (112mEq/l), but the values of the other brother (22mEq/l and 105mEq/l, respectively) were normal. When an acidification test with ammonium chloride was applied, urinary pH decreased to 5.5 and 5.4 respectively, therefore, sensu stricto, this could only be incomplete distal tubular acidosis.2 Perhaps the first of the brothers had a proximal bicarbonate loss. Nevertheless, treatment with alkali salts did not affect the levels of serum magnesium. In the third of these first articles, it is reported that the affected brothers had incomplete tubular acidosis and hypocitraturia, moderate renal failure and data similar to that mentioned above.3 It is striking that some of the first patients were suffering from acute pyelonophritis.2,3
In 1979 the first article appeared in which hypercalciuria with bilateral macular coloboma are related.4 Its authors believed they were facing a new variant of oculo- renal syndrome, since there were some commonalities with Lowe syndrome. One of the two brothers, members of a consanguineous family, also had a moderate proximal tubular loss of bicarbonate. To this was added a coloboma with tapeto-retinal degeneration.
That same year, at the National Meeting of Paediatric Nephrology (Oviedo, 1979), the Zaragoza Group presented the first Spanish cases of “Idiopathic renal hypercalciuria Association with macular coloboma”.5 It was suspected that the hypercalciuria was of renal origin because calcium/creatinine ratio was unchanged after oral calcium overload. The children, in addition to coloboma, had myopia and nystagmus.
In the eighties to mid –nineties it must be noted that many articles on this entity were published in Spain, something very praiseworthy as this condition is rare. These papers were on both children and adults.6-12 However, reports continued, Gil- Gibernau in 1982, a member of the Section of Paediatric Ophthalmology of the Vall d’ Hebron Hospital in Barcelona, was first author of an article on four cases of infantile idiopathic hypercalciuria associated with bilateral congenital myopia and atypical macular coloboma. Magnasaemia was not determined in these patients, which, on the other hand, suffered from nephrocalcinosis and concentration capacity deficit.13 The paper was important, and it was mentioned as a new condition in the second edition of the famous book on Pediatric Nephrology edited by Edelmann Jr. Gil- Gibernau syndrome was mentioned as a new conditions with associated hypercalciuria, myopia and macular coloboma.14
THIS DISEASE IS CHARACTERISED AS BEING A DISTINCT TUBULOPATHY AND IS GIVEN ITS FINAL NAME. ALL THIS, BY SPANISH AUTHORS
Hypomagnesaemia of renal tubular origin was properly classified in the late eighties. Juan Rodríguez Soriano et al., in 1987, described in an article that at least three hereditary causes of hypomagnesaemia should be considered, all caused by corresponding defects in renal tubular magnesium reabsorption. These are: familial isolated hypomagnesaemia, familial hypomagnesaemia-hypopotassaemia or Gitelman syndrome, frequently confused with Bartter syndrome, and familial hypomagnesaemia-hypercalciuira, with which 15 patients had been described, to which the authors added three new cases.15 As to this last entity, the authors wrote that “hypomagnesaemia was always accompanied by hypercalciuria and nephrocalcinosis. Ocular abnormalities such as myopia and horizontal nystagmus are often present. Hypermagnesiuria is of greater importance than in previous entities and reflects a reduced magnesium reabsorption Tm. The defect must be located in the ascending limb of the loop of Henle and involve both calcium and magnesium transport. Indeed, shortly after it was demonstrated that this defect was located where Rodriguez Soriano et al. had predicted. A few years later, the same authors studied the acidification defect in this tubulopathy.16 Tests to stimulate the ability of distal acidification showed a decrease in urinary pH (4.7 to 5.6), but ammonia excretion was reduced (26-46umol/min per 1.73m2, 35-54umol/100µml glomerular filtration rate). In addition, urinary pCO2 did not increase sufficiently after an overload of sodium bicarbonate (pCO2 urine/blood gradient: 1.3-17.7mHg). The authors argued that this disruption in the ability to achieve distal acidification could be explained by a defect in the tubular transfer of ammonia and also hydrogen ion secretion in the medullary collecting tube, probably caused by a medullary interstitial nephropathy.
In 1995, Manuel Praga et al. published clinical and biochemical data corresponding to eight patients from five families. The authors found that in this tubulopathy, of autosomal recessive inheritance, there was progressive deterioration of renal glomerular function and tubular damage that did not relapse after trasplant.12 The title of this article endured and gave the disease its final name, i.e. “Familial Hypomagnesaemia with Hypercalciuria and Nephrocalcinosis”.
IT WAS DISCOVERED THAT FAMILIAL HYPOMAGNESAEMIA WITH HYPERCALCIURIA AND NEPHROCALCINOSIS IS THE FIRST TUBULOPATHY CAUSED BY DYSFUNCTION OF SOME PROTEINS THAT ARE EXPRESSED IN THE PARACELLULAR space OF THE ASCENDING LIMB OF HENLE’S LOOP
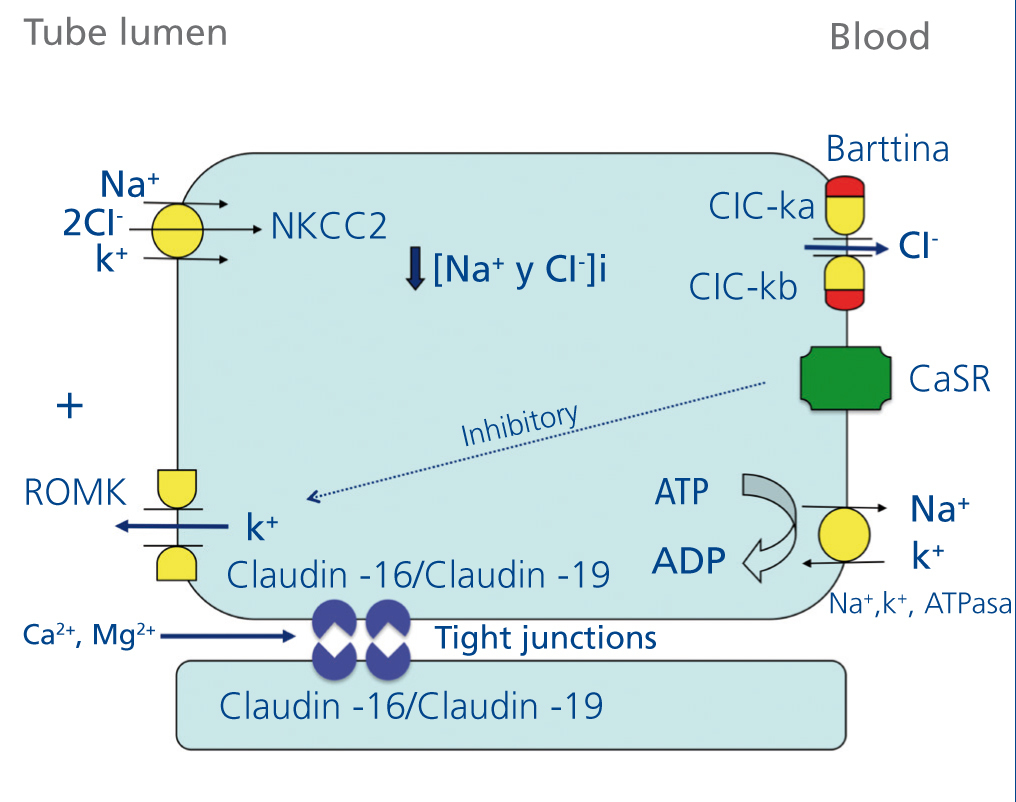
From the beginning of the seventies it was known, thanks to micropuncture studies, that magnesium is predominantly reabsorbed in the ascending limb of Henle’s loop.17,18 In 1982, Shareghi and Agus showed that in this tubular segment, the presence of a positive transepithelial voltage is an important force favouring net reabsorption of magnesium, calcium and potassium19 (Figure 1). In addition, also, by micropuncture studies, it was revealed that significant amounts of magnesium (50-60%) are reabsorbed in the thick ascending limb of Henle’s loop compared to sodium (20-25%) or calcium (30-35%).20 In the early nineties there was sufficient information to know that magnesium is reabsorbed by the paracellular route 20 21 (Figure 1). However, it was not yet known if this reabsorption was favoured by the action of certain proteins. Indeed, in 1999, Simon et al. described the existence of a protein, paracellin-1, necessary for paracellular tubular reabsorption of manesium.22 This protein is located in the tight junction areas. i.e. intercellular structures that allow tight contact between adjacent epithelial cells. In this same study performed by Simon et al, it was found that mutations of PCLN -1 gene encoding paracellin-1 were the cause of FHHNC.22, 23 Once it was demonstrated that paracellin-1 was a member of the claudin family, it was renamed claudin-16 (CLDN16 gene). It is worthwhile mentioning, that the name claudin is a tribute to Dr. Philippa Claude, who in the seventies, with the aid of an electron microscope, studied the intercellular structures known as “zonula occludens”.24
When it was thought that everything worth knowing about the disease was known, two new features required explanation. Firstly, Loris et al.’s description, based on a series of nine cases, which mentioned that, in Spanish patients, the frequency of eye alterations was unusually high (81% in comparison with 24% in patients from other countries).25 Second, the observation that there are patients with FHHNC that do not have any CLDN16 gene mutations. In this respect, it was striking that the Spanish patients with FHHNC, with the exception of a few,22, 26 did not have mutations in this gene.27
In 2006, Konrad et al. resolved the matter determining that patients with CLDN19 gene mutations, another claudin multigene family member, suffered from hypomagnesaemia, chronic kidney disease (CKD) and severe ocular abnormalities.28 The product of the gene, claudin-19, carries out its functions, in the same way as claudin-16, at the site of the tight joints of renal tubules and the retina.
A year later, it was found that, under physiological conditions, claudin-19 acts as a selective barrier to cations in tight junctions and regulates the permeability for monovalent and divalent cations.29 In 2008, Hou et al. demonstrated that claudin-16 interacts with claudin-19,30 in such a way that this association confers on tight junctions the capacity to contain a selective cation reuptake mechanism.31
MOST SPANISH PATIENTS WITH FAMILIAL HYPOMAGNESAEMIA WITH HYPERCALCIURIA AND NEPHROCALCINOSIS ARE CARRIERS OF THE SAME CLDN19 GENE MUTATION.19 FOUNDER EFFECT
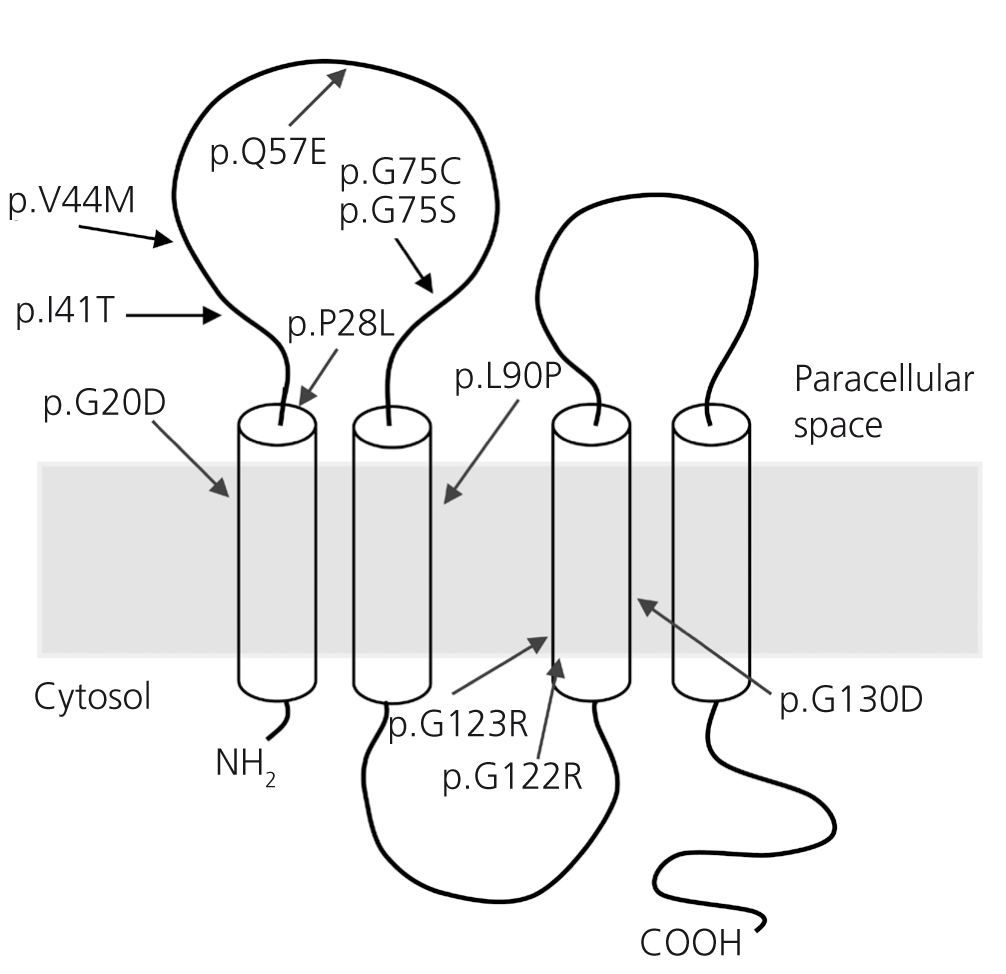
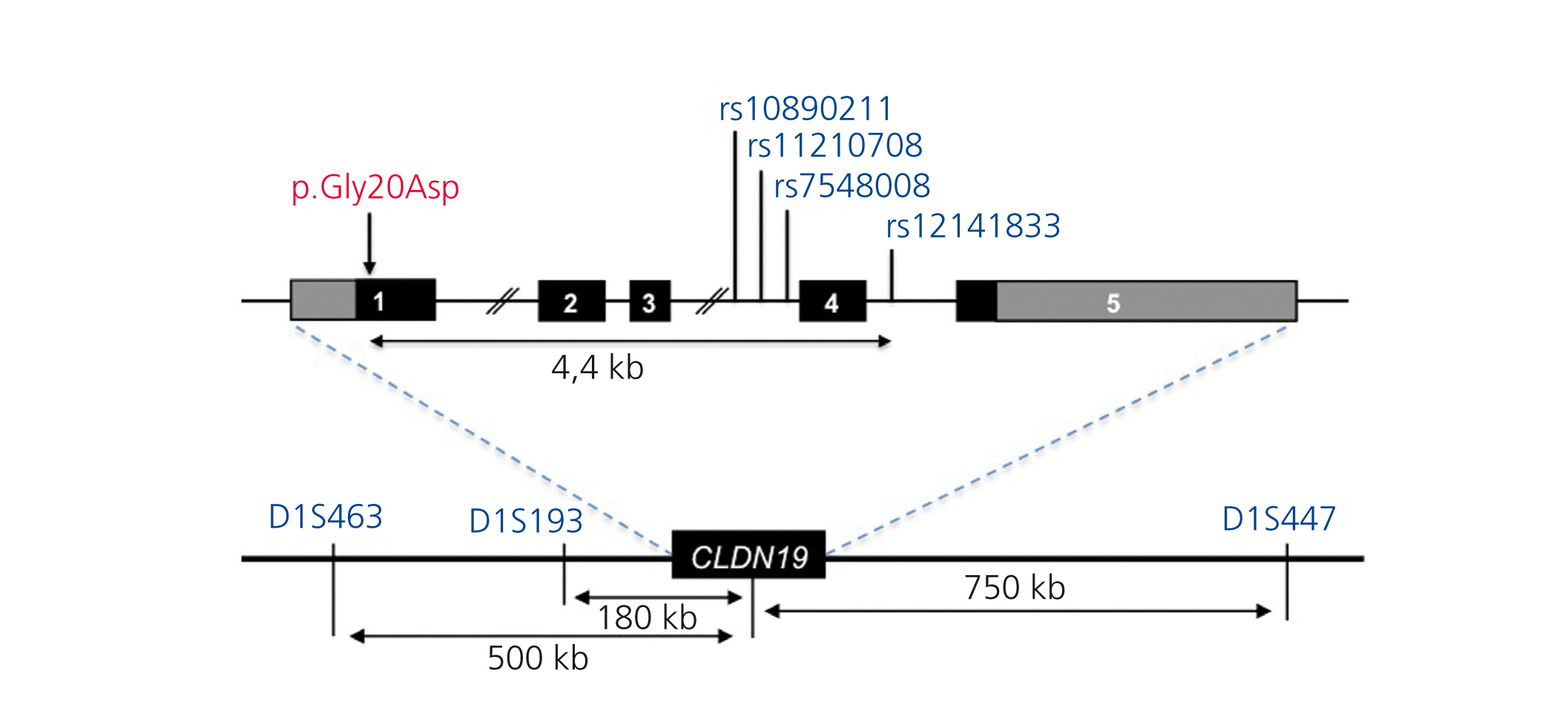
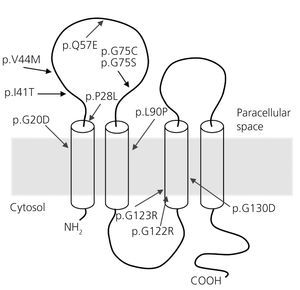
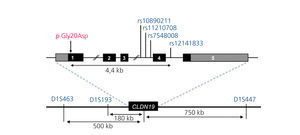
To date only few mutations have been described in the CLDN1928,32,33 gene (Figure 2). The original study by Konrad et al. included seven Spanish patients from different families in which the same homozygous mutation, p.G20D (c.59G > A), was detected in the CLDN19 gene, which they called Spanish/Hispanic mutation.28 The mutation results in the replacement of glycine by aspartic acid at position 20 of claudin-19 that alters the signal peptide causing the mutant protein to be retained within the cell and not to reach its normal location, which is the cell membrane where the normal protein is physiologically located.28,30 Konrad et al. suggested that p.G20D is a founder mutation. i.e. that it originated in a common founder ancestor. The other alternative is that the mutation appeared on a site prone to mutate (hot spot), in which case individuals with the mutation may not be related and surrounding DNA sequence varies. Conversely, when it is a founder mutation, it is located in a region of the chromosome identical to that seen in the “founder subject.” Vargas-Poussou et al. published a study that described two unrelated Spanish patients and 13 French patients, belonging to 12 families, with p.G20D mutation, mostly in homozygosis.32 Families from southwest France were of Spanish origin. The data obtained by microsatellites analysis in these families are consistent with a founder effect.32 Subsequently, our group has published the results of a Spanish cohort of 34 patients with FHHNC, 20 girls and 14 boys, members of 30 apparently non-related families.33,34 Clinical data of these patients show a high risk of progression to end-stage CKD (62% with impaired renal function and 20% with transplants), consistent with what was observed in the series of French patients.32 Our results are also consistent with those seen in the French group with high percentages, 88%, and 91%, respectively, of patients with severe eye anomalies such as myopia magna, nystagmus and coloboma. In contrast, nine patients with mutations in the CLDN16 gene studied by the French group, most of which came from North Africa, did not have ocular abnormalities. Mutation analysis in our cohort showed that all patients have mutations in the CLDN19 gene. The p.G20D mutation was detected in 93% of unrelated patients and was present in homozygosis in 83 % of cases.33,34 None of our patients showed mutations in CLDN16. Indeed, as indicated above, we only know of the existence of three Spanish patients with mutations in this gene.22,26 In our study, we determined the haplotypes of patients and relatives by genotyping three microsatellites (D1S463, D1S193, D1S447) that surround the CLDN19 locus used previously in the French cohort32 and four single nucleotide polymorphisms (SNPs, rs10890211, rs11210708, rs7548008, rs12141833) located in introns 3 and 4 of the gene33,34 (Figure 3). The results of these analyses showed that alleles with the mutation p.G20D exhibit a common haplotype which is inherited with this. Our results, together with previous results from other Spanish patients or patients of Spanish origin, indicate the existence of a founder effect responsible for FHHNC in Spain. The region around mutation p.G20D shared by different patients is relatively large (about 500 to 1000kb), so we can assume that the founder mutation originated recently, perhaps a few hundred years ago in a common ancestor of all these families. The fact that most of our patients present the same mutation in the CLDN19 gene facilitates molecular diagnosis of the disease in our country. The analysis of mutations p.G20D should be the first genetic screening in patients with FHHNC.
The pathogenesis of CKD in patients with FHHNC is unknown. It has not been possible to establish a correlation between the degree of nephrocalcinosis and progression to chronic renal failure. However, it has been suggested that the effect of CLDN16 mutations on claudin-16 function takes place during kidney development, and that, therefore, early progression to CKD may be due to abnormal kidney development followed by fibrosis and nefrocalcinosis.32,35 The results of a study in a group of 71 patients with mutations in CLDN16 showed that patients with mutations that lead to a complete loss of function of claudin-16 in both alleles exhibit symptoms of the disease at a younger age than those with, at least, one mutant allele with partial function; furthermore, patients with mutations with complete loss of function rapidly progress to CKD35; i.e. residual function of some claudin-16 mutants delays progression to CKD. Compared with CLDN16, in which 40 different mutations have been identified, only 13 mutations have been described in CLDN19. No genotype-phenotype correlations in patients with mutations in the latter gene have yet been published. Recent findings suggest that patients FHHNC with mutations in CLDN19 have a greater risk of developing CKD than patients with mutations in CLDN1632. However, these results should be treated with caution, since most of the patients of the first group are carriers of mutation p.G20D that causes a complete loss of function.
With regards to extrarenal symptoms, patients with CLDN19 mutations suffer from, in the majority of cases, severe eye abnormalities not seen in patients with CLDN16 mutations.28,32,35 This difference may be explained by the fact that claudin-19 is also expressed, in a prominent manner in the pigmented epithelium of the foetal retina, whereas expression of claudin-16 in said epithelium is low.28,36 Furthermore, in a few patients with CLDN19 mutations neuromuscular disorders have been described.32,34 These have been related to behavioural anomalies seen in mouse models deficient in caludin-19.37 Claudin-19 is also expressed in cell joints in Schwann cells, therefore mutations in the coding of this gene may cause peripheral nervous system deficiencies.37
Acknowledgements
Research in our group has been funded by the Health Research Fund (Projects PI09/91009 and PI11/00342, cofinanced by the European Regional Development Fund). The authors are members of the group RenalTube (www.renaltube.com).
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Schematic representation of ion transport in the thick ascending limb of Henles loop
Figure 2. Mutations described in the CLDN19 gene (28,32,33) and its location in claudin-19 model.
Figure 3. Chromosome 1p34.2 region containing the locus CLDN19.