El fosfato no goza de buena reputación entre los nefrólogos. Se asocia con la calcificación vascular y con una gran mortalidad cuando sus niveles en sangre son elevados. Provoca daños en las células endoteliales vasculares y transformación osteoblástica en las células de los músculos lisos vasculares en cultivos. Aunque muchos de los resultados adversos en los pacientes con enfermedad renal crónica (ERC) se atribuyen al fosfato, se desconoce el mecanismo molecular preciso que hay detrás de los problemas que causa. Este déficit de conocimiento ha limitado las opciones terapéuticas a la restricción del fosfato en la dieta, a captores del fosfato y a la eliminación forzada del fosfato a través de diálisis. El objetivo de esta revisión es llamar la atención de los nefrólogos sobre un metabolito del fosfato denominado partículas de calciproteínas (PCP). Se está discutiendo la posibilidad de las PCP como patógeno y nuevo objetivo terapéutico de la ERC.
Phosphate is a “bad guy” among nephrologists. Phosphate is associated with vascular calcification and high mortality when it is elevated in the blood. It induces damages to vascular endothelial cells and osteoblastic transformation in vascular smooth muscle cells in culture. Although people believe that many adverse outcomes in patients with chronic kidney disease (CKD) are attributed to phosphate, nobody knows the precise molecular mechanism behind these phosphate woes. This knowledge gap has limited therapeutic options to dietary phosphate restriction, phosphate binders, and forced removal of phosphate by dialysis. The purpose of this review is to call nephrologists’ attention to a phosphate metabolite termed calciprotein particles (CPPs). The possibility of CPPs as a pathogen and a novel therapeutic target of CKD is discussed.
Since hyperphosphatemia was identified as a potent mortality risk,1,2 phosphate binders have been prescribed for CKD patients to lower serum phosphate levels, and have indeed improved their clinical outcomes.3 Because serum phosphate levels stay within normal range until CKD advances to stage 4-5, the use of phosphate binders is justified for end-stage renal disease (ESRD), which accounts only for a few percent of total CKD patients.4,5 However, all-cause mortality is known to correlate positively with serum phosphate levels even when they are within normal range.6 These observations have evoked a fierce debate on whether or not indication of phosphate binders should be expanded from ESRD to moderate CKD patients in order to further lower their normal serum phosphate levels.
Several clinical studies have been performed to determine whether or not phosphate binders are beneficial for moderate CKD. Block et al. reported a prospective randomized study of 148 CKD patients at stage 3-4 to determine the effect of three different phosphate binders on vascular calcification.7 The result was somewhat unexpected: Phosphate binders rather accelerated vascular calcification. However, when the effect of different binders (calcium acetate, lanthanum carbonate, and sevelamer carbonate) was analyzed separately, calcium acetate was found primarily responsible for the adverse outcome, whereas the other calcium-free binders were not statistically different from placebo, at least in the small number of patients treated for up to 9 months. Further studies are necessary to conclude that phosphate binders are not beneficial for moderate CKD. Of note, several other studies found advantage of calcium-free binders over calcium-containing binders in suppressing vascular calcification and all-cause mortality in dialysis patients as well as stage 3-4 non-dialysis CKD patients.8-13 This may be because calcium-containing binders cause calcium overload, which may contribute to vascular calcification. In fact, Hill et al. reported that calcium carbonate induced positive calcium balance in stage 3-4 CKD patients.14
How can all these observations be reconciled? Why is phosphate harmful and how does calcium affect the phosphate woes? As a plausible hypothesis that potentially addresses these questions, I have proposed that cardiovascular complications in CKD are triggered by a pathogen called CPP.15
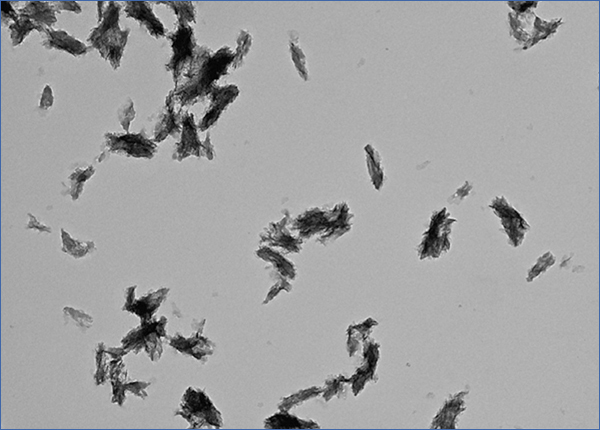
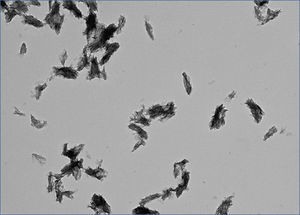
What is CPP? CPP stands for calciprotein particles, which are nanoparticles composed of calcium-phosphate (CaP) crystals and mineral binding proteins such as Fetuin-A. The process of CPP formation has been studied extensively in vitro.16-19 When concentration of calcium and phosphate exceeds the solubility limit, insoluble CaP crystals are generated instantaneously. They can grow over time and eventually precipitate as hydroxyapatite. However, CaP crystals do not grow in the blood, because serum protein Fetuin-A absorbs CaP crystals and prevents them from growing into large precipitates. The CaP crystal-laden Fetuin-A molecules aggregate to form nanoparticles or CPP (Figure 1). Because CPPs are nanoparticles, they are dispersed in the serum as colloid particles and not precipitated. Thus, formation of CPP can be regarded as a defense mechanism that prevents blood vessels from being occluded with insoluble CaP precipitates. Solubilizing insoluble substance as colloid particles using its binding protein is a universal strategy typically seen in lipoporteins.
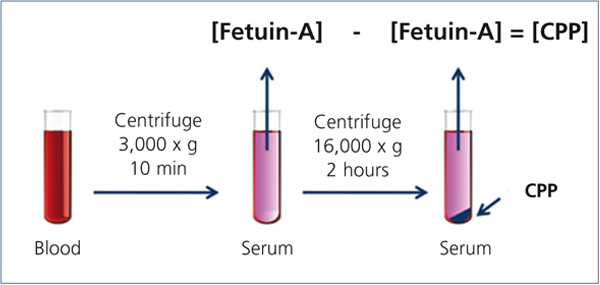
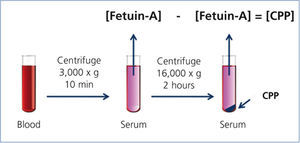
Recent studies have indicated that CPPs appear in the blood of CKD patients.20,21 In these studies, serum CPP levels were measured by a “sequential centrifugation” method: First, clotted blood was centrifuged at 3,000 x g for 10 minutes to harvest serum. Because CPPs are colloid particles, they never precipitate under this condition and stay in the serum. Next, the serum was centrifuged at a higher speed (16,000-22,000 x g) for a longer time (2 hours) to precipitate CPPs. They measured serum Fetuin-A concentration by ELISA before and after the high-speed centrifugation and assumed that the difference of Fetuin-A concentration between the two represented the serum CPP level (Figure 2). These studies have shown that serum CPP levels were positively correlated with coronary calcification score20 and independently associated with serum phosphate, inflammation (high-sensitive CRP), procalcific factors (oxidized LDL and BMP-2/7 ratio), arterial stiffness (aortic pulse wave velocity), and decline of renal function (eGFR).21 Importantly, many of these findings were observed in stage 3-4 CKD patients whose serum phosphate levels were within normal range.
On the other hand, numerous basic studies have described the effect of high extracellular phosphate on various types of cells in culture. When phosphate was added to the tissue culture medium, oxidative stress and cell death were induced in vascular endothelial cells.22,23 In vascular smooth muscle cells, phosphate induced phenotypic transition into osteoblastic cells, which was associated with induction of bone-related gene expression including BMP-2, RUNX2, and osteopontin.24,25 These cellular responses to high extracellular phosphate, if occurred in vivo, may explain vascular calcification in ESRD.26 However, it should be noted that phosphate and calcium concentration in regular tissue culture media like DMEM is about 1mM and 2mM, respectively, which is close to the solubility limit. Thus, a small increase in phosphate or calcium concentration potentially triggers formation of CaP crystals and, in the presence of serum, leads to formation of CPPs. Thus, it is possible that the effect of phosphate on cultured cells may actually be attributed to CaP crystals or CPPs but not to phosphate itself. In fact, some studies tested this possibility and demonstrated that this was indeed the case: Phosphate failed to induce those cellular responses when formation of CaP crystals was blocked with pyrophosphate or phosphonoformic acid.27-29
Taken together, a plausible scenario is that serum CPPs function as a ligand that triggers damages in vascular endothelial cells and osteoblastic transition in vascular smooth muscle cells, thereby leading to vascular calcification in CKD. In other words, CPP may be regarded as an endogenous “pathogen” that circulates in the blood and causes vascular calcification. Because serum CPP levels can be high even when serum phosphate levels are within normal range,21 this hypothesis explains why vascular calcification occurs in CKD patients whose serum phosphate levels are not elevated. It also explains why calcium-free binders tend to be more beneficial than calcium-containing binders.
Many questions must be addressed before this “CPP theory of CKD” is verified: Where do serum CPPs come from? The fact that CPPs are found in the serum of CKD patients with normal serum calcium and phosphate levels has raised the possibility that formation of CaP crystals (nucleation) may not necessarily take place in situ in the blood. It is possible that CaP crystals generated somewhere else may enter the blood stream and bind to Fetuin-A to become CPPs. Then, where is the place of nucleation and why is nucleation accelerated in CKD patients? Obviously it is of critical importance to identify the cell-surface receptor for CPPs. If these questions are addressed and the CPP theory of CKD is verified, CPP may be justified as a diagnostic marker for vascular calcification in CKD. Recently, a new assay was reported, which measured a physical property of serum CPPs as colloid particles in vitro to determine the overall calcification propensity of serum.30 In fact, the result of this assay was independently associated with aortic stiffness and mortality.31 In addition, CPP may be justified as a novel therapeutic target. Suppression of CPP formation or action by inhibitors for CaP crystal formation or putative CPP receptor may become a novel strategy for the treatment of CKD.
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Figure 1. Calciprotein particle.
Figure 2. Measurement of the serum CPP level.