La enfermedad renal crónica (ERC) y la diabetes mellitus tipo 2 (DM2) son afecciones crónicas de elevada prevalencia que representan un importante problema de salud pública y requieren un abordaje interdisciplinario. La DM2 es la principal causa de ERC en nuestro medio y también constituye una importante comorbilidad de la nefropatía no diabética. Los pacientes con diabetes e insuficiencia renal son un grupo de especial riesgo, pues presentan una mayor morbimortalidad y un superior riesgo de hipoglucemias que los sujetos diabéticos con función renal normal. El tratamiento de la DM2 en los pacientes con ERC resulta controvertido dada la escasez de evidencias disponibles. El presente documento de consenso pretende facilitar la adecuada elección y dosificación de los fármacos antidiabéticos y el establecimiento de unos objetivos seguros de control glucémico en los pacientes con ERC.
Chronic kidney disease (CKD) and type 2 diabetes mellitus (T2DM) are highly prevalent chronic diseases that represent a significant public health problem and require multidisciplinary management. T2DM is the main cause of CKD in our setting and it is also a major comorbidity of non-diabetic nephropathy. Patients with diabetes and renal failure represent a special risk group as they have higher morbidity and mortality and are at a higher risk of hypoglycaemia than diabetic individuals with normal renal function. Treatment of T2DM in patients with CKD is controversial because of the scarcity of evidence available. This consensus document aims to facilitate the appropriate selection and dosage of anti-diabetic drugs as well as establishing glycaemic control safety targets in patients with CKD.
INTRODUCTION
Epidemiology
Chronic kidney disease (CKD) and type 2 diabetes mellitus (T2DM) are very prevalent chronic diseases that constitute a major public health problem, they generate a high consumption of resources and require appropriate coordination of the various professionals involved in their treatment.1,2
T2DM has become pandemic.1,3 The prevalence of diabetes in Spain has been estimated at almost 14% of the adult population.4 Likewise, CKD is a problem emerging all over the world. In Spain, the Epidemiology study of Chronic Renal Failure in Spain, EPIRCE, estimated that around 10% of the adult population would suffer some degree of CKD.50
Diabetes is a major modifiable risk factor for developing CKD. T2DM is the main cause of CKD and it has high morbidity in non-diabetic nephropathy. It has been estimated that 27.9% of patients with T2DM in Spain have CKD6 and that more than 35% have microalbuminuria, proteinuria or CKD.7 According to these data, there would be almost 2 million people with diabetes and various degrees of renal involvement in Spain. Studies carried out in different countries have found that in a population with T2DM, the prevalence of microalbuminuria (the earliest manifestation of diabetic nephropathy) and proteinuria is 27%-43% and 7%-10% respectively.8-10 The prevalence of proteinuria increases significantly from 15 years after diabetes is diagnosed.11 The presence of albuminuria in patients with T2DM is a predictor of chronic renal failure, with the mean time from the start of proteinuria until end-stage kidney disease being 7 years.12 The risk of renal failure is 25 times higher in diabetic patients than in the non-diabetic population.7 In Spain, 22% of T2DM patients show a decrease in their glomerular filtration rate (GFR) to less than 60ml/min/1.73m2.13 According to data maintained by the Spanish Society of Nephrology (S.E.N.) corresponding to 2010, diabetes is the main cause of advanced CKD in Spain and is responsible for 24.7% of renal replacement therapy cases, although in some regions, such as the Canary Islands, this figure is as high as 45%.14 In our country, patients with diabetes and CKD are older and have higher cardiovascular morbidity (dyslipidaemia, ischaemic heart disease or peripheral vascular disease) than in the non-diabetic population with CKD,15 as well as higher mortality, which in 49% of cases is due to a cardiovascular cause (non-published data corresponding to the S.E.N. MERENA study).<0}
Estimation of renal function and classification of chronic kidney disease
Given that T2DM is a risk factor for developing CKD and that the prevalence of hidden or undiagnosed CKD is very high, kidney function screenings have been recommended at least once a year, by determining the GFR and measuring albuminuria in all T2DM patients.16,17 Estimating the GFR is more reliable for assessing kidney function than measuring plasma creatinine, particularly in diabetes patients. The 2012 Kidney Disease Global Outcomes (KDIGO) guidelines18 recommend using the Chronic Kidney Disease Epidemiology (CKD-EPI) formula.19
The KDOQI guidelines20 define CKD as the presence of a GFR of less than 60ml/min/1.73m2 for at least 3 months or structural lesions in the kidney (histological abnormalities in the renal biopsy) or functional issues (albuminuria, urinary sediment abnormalities or imaging test abnormalities), which could potentially cause a decrease in the GFR.
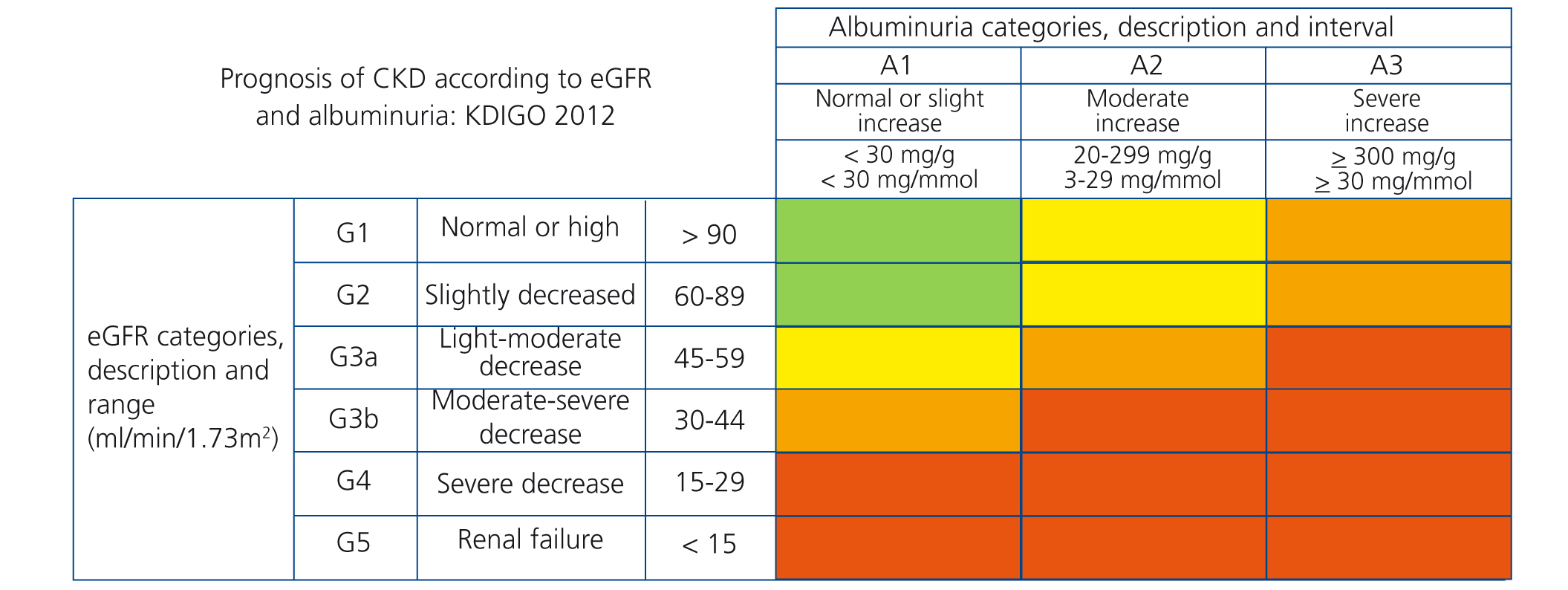
The new prognostic classification of CKD proposed by KDIGO18 is based on albuminuria and glomerular filtration stages (Figure 1).
The consensus document on CKD signed by the S.E.N. and the Spanish Society of Family and Community Medicine in 2008 established that the early diagnosis of hidden CKD is important, especially in diabetes patients, in order to decrease morbidity, CKD progression and mortality in these patients.16
TREATMENT OF VASCULAR RISK FACTORS IN PATIENTS WITH DIABETES AND CHRONIC KIDNEY DISEASE
Prognostic importance of chronic kidney disease in diabetes patients
Diabetic nephropathy is a major marker of morbidity and mortality in diabetes patients. Microalbuminuria and a decrease in the GFR below 60ml/min/1.73m2 are considered main factors of cardiovascular risk in the Joint National Committee report21 and of subclinical target organ damage in the European guidelines of the European Society of Hypertension and the European Society of Cardiology,22 respectively.
In the Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE) trial, it was observed that, as albuminuria increased and the estimated GFR decreased, the T2DM patient had more cardiovascular events, and as such, for an albumin/creatinine ratio >300mg/g and an estimated GFR of 60ml/min/1.73m2, the risk of suffering a cardiovascular or renal event was 3.2 and 22 times higher, respectively, than in patients in whom both values were normal.23
The presence of proteinuria in diabetes patients, even when the GFR is normal, is a strong indicator of kidney disease progression and mortality.24,25 Macroalbuminuria is a better predictor of the rate of renal deterioration than the baseline GFR.26 The rate of renal deterioration is also higher in older diabetic patients.27
CKD is associated with a marked increase in cardiovascular episodes (myocardial infarction, chronic heart failure, stroke, peripheral arterial disease) generically included in the cardio-renal syndrome type 4.28 The European Guidelines recently considered CKD (defined as a GFR less than 60ml/min/1.73m2) to be a coronary heart disease risk equivalent.29
Blood pressure control targets in a patient with diabetes and chronic kidney disease
High blood pressure is a factor involved in CKD progression along with proteinuria and poor control of carbohydrate metabolism. In CKD patients, the objective of antihypertensive treatment is threefold: to reduce blood pressure, reduce the risk of cardiovascular complications and delay CKD progression. A systematic review estimates that only 12% of hypertensive diabetes patients have good blood pressure control,30 although recent data indicate a favourable trend in high blood pressure control. In Spain, in the 2010 PRESCAP study,31 conducted in Primary Care on a population of almost 13,000 hypertensive patients that included 31% of subjects with diabetes, almost 50% of patients had adequate blood pressure control. Diabetes patients frequently have non-diagnosed nocturnal hypertension, which could in part explain the excessive cardiovascular risk in some patients. Furthermore, in normotensive diabetic patients with years of progression, masked hypertension must be ruled out, which may be present in up to 29% of cases.32 As regards hypertensive diabetic patients, 4.9% of those who have good blood pressure control at the clinic have poor control in ambulatory blood pressure monitoring (ABPM).33 As such, the routine use of ABPM with protocol should be considered in diabetes patients, particularly if they have CKD.
In general, clinical blood pressure figures <140/90mmHg are recommended in CKD patients.26,34 However, the presence of diabetes may make it advisable to set a rather lower blood pressure target. The recent European Blood Pressure Guidelines35 sets a general systolic blood pressure target of <140mmHg for all patients, even for high-risk subjects, including those with diabetes and CKD. A more flexible target of 140-150mmHg has been proposed for the elderly. The American Diabetes Association recommends a general blood pressure control target of <140/80 mmHg36 in diabetes patients.
Lipid control targets in patients with diabetes and chronic kidney disease
One of the factors that accelerate the deterioration of renal function is dyslipidaemia, independently of its arteriosclerosis promoting effect.
In accordance with the latest European Guidelines,29 CKD subjects must be considered high or very high cardiovascular risk patients, without the requirement for risk scales. As such, the presence of CKD with a GFR <60ml/min/1.73m2 classifies the patient as a coronary heart disease risk equivalent and establishes a target of c-LDL <70mg/dl or a reduction of 50% if the previous target is not achievable.
Data obtained for post-hoc analysis support the capacity of statins to reduce cardiovascular complications in patients with stages 2 and 3 CKD.37,38 The results in stages 4 and 5 CKD, or in haemodialysis are not as clear.39,40 Nevertheless, in the SHARP study, which included a large number of diabetes patients, we observed a 17% reduction in cardiovascular episodes in stage 3, 4 and 5 CKD subjects, treated with ezetimibe/simvastatin versus placebo. This reduction was not observed in dialysis patients.41
The drug of choice is statins, alone or in combination with ezetimibe. Statins with poor renal elimination, such as atorvastatin and fluvastatin, do not require dose adjustment in CKD patients. Simvastatin and pravastatin doses should be reduced in patients with a GFR <30ml/min. According to its data sheet, rosuvastatin does not require a dose adjustment with a GFR >60ml/min, but half doses should be used if the GFR is <60ml/min and it is contraindicated in patients with advanced CKD. Pitavastatin should be used with caution in patients with moderate to severe renal failure and maximum doses should be avoided in these cases. Ezetimibe does not require a dose adjustment.
To treat severe hypertriglyceridaemia, fibrates and omega-3 fatty acids are used. Most guidelines recommend gemfibrozil as the fibrate of choice, although its use is not recommended if the GFR is <15 ml/min.
It must be borne in mind that the risk of rhabdomyolysis due to statins increases in CKD patients. The risk is more than 5 times greater when statins and fibrates are combined (in this situation fenofibrate should be used instead of gemfibrozil), and therefore this dual treatment should be used with caution and be strictly monitored.
Antiplatelet therapy in patients with diabetes and chronic kidney disease
The KDIGO guidelines18 recommend the use of antiplatelet therapy in CKD patients at risk of atherosclerotic complications whenever their risk of bleeding does not surpass the expected benefit. This recommendation, which has been extended to diabetes patients, is difficult to apply clinically whenever the subject has a GFR <60ml/min, given that in these cases, both conditions apply (more frequent atherosclerosis and risk of bleeding due to renal failure), and as such, it is particularly important to individualise this indication and ensure that the blood pressure is well controlled (<140/90mmHg). If it is used, a dose of 100mg/day of aspirin should not be exceeded.
TREATMENT OF hyperglycaemia IN PATIENTS WITH CHRONIC KIDNEY DISEASE
Assessment of glycaemic control in chronic kidney disease patients
Glycated haemoglobin (HbA1c) is the reference parameter for assessing metabolic control in CKD patients, although there are circumstances that limit its precision. On the one hand, uraemia promotes the formation of carbamylated haemoglobin, which interferes in the measuring of HbA1c when it is measured by high-pressure liquid chromatography, resulting in falsely high levels. On the other hand, other factors could cause a false decrease in HbA1c levels, such as the lower half-life of red blood cells, transfusions and an increase in erythropoiesis after treatment with erythropoietin.42-47
This false decrease in HbA1c values and the lack of correlation with glycaemia levels are observed particularly in patients on haemodialysis who are receiving erythropoiesis-stimulating agents.48 Although some authors advise using glycated albumin measurements as a method of assessing glycaemic control,49,50 this is not the position accepted by the majority. The alternative in these cases would be to carry out frequent capillary glucose tests.
Hypoglycaemia and chronic kidney disease
CKD is a risk factor for developing hypoglycaemia. Patients with diabetes and CKD have twice the risk of suffering from severe hypoglycaemia than those without CKD.51
In CKD, various hypoglycaemia-predisposing circumstances coincide. Most anti-diabetic drugs are eliminated via the kidneys, and as such, their half-life increases in CKD patients. In addition, insulin is cleared via the kidney, and as such, requirements for it are usually reduced in the presence of renal failure (GFR <60 ml/min/1.73m2)52; furthermore, insulin degradation in peripheral tissues decreases in CKD patients.53 Lastly, uraemic patients frequently have a decreased appetite, malnutrition and a reduction of liver glycogen depositions,54 and renal gluconeogenesis decreases as the renal mass is reduced.53,55 The risk of severe hypoglycaemia may be particularly high in dialysis patients and in those who suffer from autonomic neuropathy, in whom adrenergic warning symptoms are usually absent.
Intensive treatment of T2DM is associated with an increase in the risk of severe hypoglycaemia.56 Furthermore, The Action to Control Cardiovascular Risk in Diabetes (ACCORD)57 and ADVANCE58 studies and Veterans Affairs Diabetes Trials (VADT)59 have demonstrated that severe hypoglycaemia is a marker of cardiovascular and total mortality in T2DM patients.
As a result, when planning anti-diabetic treatment in CKD patients, it is very important to minimise the risk of hypoglycaemia episodes by establishing safe glycaemic control objectives and a suitable choice and dose of anti-diabetic drugs.
Glycaemic control targets in chronic kidney disease patients
One of the main decisions in addressing T2DM is establishing the glycaemic control targets. The need to individualise HbA1c is increasingly stressed, but no methods have been established to apply specific individualisation criteria for CKD patients.
The United Kingdom Prospective Diabetes Study (UKPDS)60 demonstrated that intensive glycaemia treatment (HbA1c 7.9% versus 7%) reduced microvascular complications in general by 25%, microalbuminuria by 33% and progression to proteinuria by 39%. From this study, the general target of achieving HbA1c below 7% was established. However, the UKPDS study was performed in patients with T2DM, mostly without cardiovascular or kidney disease, and as such, its conclusions cannot be applied to patients with CKD.
The ADVANCE study,58 conducted on T2DM patients with at least one vascular risk factor, found that intensive glycaemia control (HbA1c 6.5% versus 7.3% in the control group) caused a 10% reduction in the combined result of macrovascular and major microvascular complications 5 years after starting follow-up, mainly as a result of a 21% reduction of the nephropathy. There was also a significant 9% reduction in microalbuminuria.61
The ACCORD study,57 conducted on a population with T2DM progression (mean 10 years) and a history of clinical or subclinical cardiovascular disease and/or multiple risk factors, found an increase in overall mortality (22%) in the intensive control group with respect to the conventional glycaemic control group (HbA1c 6.4% versus 7.5%).
On the basis of these results, individualisation of the glycaemic control targets is currently recommended in accordance with the patient’s clinical and psychosocial characteristics.62 Nevertheless, most studies that have assessed the glycaemic control target by HbA1c did not stratify the patients in accordance with the GFR or creatinine clearance; at best, the state of renal function was assessed through plasma creatinine levels, and as such, the evidence existing in this regard is limited.
In patients with T2DM with short progression, without major comorbidities and with a low risk of hypoglycaemia episodes and a good life expectancy, it is recommended to carry out an intensive glycaemic control and achieve HbA1c of 6.5%-7%.29 This recommendation could be applicable to patients with mild CKD and T2DM (gfr >60ml/min/1.73m2), especially if they have microalbuminuria, since the strict control of glycaemia in these cases may delay the progression of the renal lesion.61
By contrast, in patients with long-term T2DM, with major comorbidities, a marked risk in episodes of hypoglycaemia, a high vascular risk or a short life expectancy, a less intensive glycaemic control is recommended (HbA1c 7.5%-8%).62 These targets could be used in moderate-advanced CKD patients (GFR <60ml/min/1.73m2), given their equivalent coronary nature,29 their high risk of hypoglycaemia episodes and the absence of evidence on the prevention of CKD progression in these cases. In frail elderly people, a more relaxed glycaemic control target may even be preferable (HbA1c <8.5%).63 However, stricter glycaemic control (HbA1c <7%) may be justified in these patients, provided that it can be achieved safely, with drugs that do not cause a risk of hypoglycaemia and that are well tolerated.
The 2005 K/DOQI guidelines49 do not establish an optimum HbA1c level for dialysis patients. Some studies with a small sample size have shown some microvascular benefits in optimising the control,50,64-66 although they have not demonstrated improved survival.67
HYPOGLYCAEMIC DRUGS IN PATIENTS WITH CHRONIC RENAL FAILURE
Metformin
Metformin is the drug of choice in the treatment of T2DM given its hypoglycaemic efficacy, its safety and low risk of inducing hypoglycaemia episodes, as well as its long-term benefits.62,68
Metformin is mainly eliminated without being metabolised, though the renal pathway via glomerular filtration and tubular secretion. Therefore, patients with renal failure are more susceptible to its accumulation and the development of lactic acidosis, a complication that may be fatal. For this reason, according to the data sheet, it should not be used in patients who have a GFR below 60ml/min/1.73m2 and the annual monitoring of renal function is advised. Nevertheless, the relationship between lactic acidosis and the accumulation of metformin is not very well documented.69 It should be noted that the risk of lactic acidosis is very low (5/100,000 patients/year) and is usually associated with decreased appetite.70 Furthermore, in the last few years, experience in the use of metformin has increased considerably, such that (on the basis of observational studies) its use is considered reasonably safe in patients with a GFR between 45 and 60ml/min/1.73m2, with renal function being monitored every 3-6 months; if the GFR is between 30 and 45ml/min/1,73 m2 it is recommended to reduce the dose of metformin by 50%, monitor renal function every 3 months and not initiate new treatment; when the GFR is less than 30ml/min/1.73 m2, metformin should be avoided.70
Renal function should always be determined before beginning treatment with metformin, and regularly afterwards, with special attention being paid to patients in situations in which renal function may change, such as in treatment with diuretics or non-steroidal anti-inflammatory drugs, or when there is a risk of dehydration (for example, in patients with dementia). Metformin should be temporarily discontinued when there is vomiting, diarrhoea or other potential causes of dehydration. When iodinated contrasts are administered, or in the event of major surgery, it is recommended to discontinue it 24 hours before or, if this is not possible, withdraw it on the day of the test or surgery and wait 48 hours to reintroduce it after checking the patient’s kidney function.71
In conclusion, we recommend to:
1. Monitor renal function before starting treatment with metformin and regularly after it is introduced, particularly in patients with risk factors for renal function deterioration (diuretics, non-steroidal anti-inflammatory drugs, iodinated contrasts, dehydration).
2. Reduce the metformin dose when the GFR is between 30 and 45ml/min/1.73m2 and do not use it whenever it is less than 30ml/min/1.73m2.
3. Temporarily discontinue metformin in the event of circumstances that put renal function at risk (vomiting, diarrhoea, radiocontrasts, major surgery).
Sulfonylureas
Given that the risk of hypoglycaemia increases markedly in CKD, sulfonylureas are not considered drugs of choice in patients with renal failure. When they are used, their metabolism and the degree of renal function should be carefully considered.53,72 The data sheets of the different sulfonylureas in the market are not very precise when they refer to their use in renal failure patients, and therefore, the consensus document recommends sulfonylurea use on the basis of their different pharmacokinetics.
Glibenclamide and glimepiride are metabolised in the liver to active metabolites that preserve hypoglycaemic action and are eliminated through urine, and as such, they accumulate in cases of CKD and may cause severe long-term hypoglycaemia. In particular, the use of glibenclamide should be avoided in patients with any degree of CKD, as is stated in its data sheet, since it is the sulfonylurea with the highest risk of hypoglycaemia.73,74 The glimepiride data sheet states that its use is contraindicated in patients with severe kidney function disorders. This consensus recommends limiting the use of glimepiride and adjusting the dose in patients with a GFR >60ml/min/1.73m2.75
After they are metabolised in the liver, gliclazide and glipizide generate inactive metabolites that are mostly eliminated through urine, and as such, there is less risk that they will cause severe hypoglycaemia. The data sheets of both drugs indicate that they can be used in patients with mild or moderate renal failure, adjusting the dose and carefully monitoring renal function.
Gliquidone is metabolised in the liver and its inactive metabolites are eliminated in bile, and as such, no dose adjustment is required and it is not contraindicated in CKD patients.76
Nevertheless, little evidence supports the use sulfonylureas in patients with severe CKD,77,78 and as such, this consensus recommends limiting its use to patients with a GFR >45ml/min/1.73m2.
Sulfonylureas bind to plasma proteins, particularly albumin, and as such, whenever necessary, they cannot be eliminated via dialysis. Some drugs (beta blockers, warfarin, salicylates, gemfibrozil, sulphonamides and thiazides) can break their bond with albumin, increasing free drug levels, with the resulting risk of hypoglycaemia.
In conclusion:
1. The risk of hypoglycaemia due to sulfonylureas increases in CKD patients, and as such, its use is generally not recommended.
2. Its use should be limited to patients with a GFR >45ml/min/1.73m2.
3. If they are used, (an adjusted dose of) gliclazide or glipizide is recommended, or gliquidone (without the need for a dose adjustment) is recommended.
Glinides
Glinides are secretagogue drugs, and as such, their use may cause hypoglycaemia.79 In contrast to sulfonylureas, glinides are metabolised in the liver, with less than 10% renal elimination, and their half-life is shorter. Although some studies have not found differences in the rate of hypoglycaemia episodes between glinides and other secretagogues,73 it is generally accepted that the risk of hypoglycaemia associated with the use of glinides is lower than with sulfonylureas.80
Repaglinade may be used with any degree of renal failure, and even in dialysis patients. In spite on this, it is recommended to start treatment with a low dose (0.5mg).
Nateglinide, despite being metabolised in the liver, is degraded to active metabolites that are eliminated by the kidney, and as such, it is not recommended for CKD patients. Furthermore, its hypoglycaemic activity is very limited.
In conclusion, repaglinade is the most recommended secretagogue for CKD patients.
Glitazones
Glitazones are metabolised in the liver and less than 2% is eliminated in urine; as a result, there is no accumulation of active metabolites in CKD. The pharmacokinetics of pioglitazone, the only glitazone currently sold in Europe, are not affected by renal function, and therefore, no dose adjustment is required, even in dialysis patients, although clinical experience is very limited in these patients.
Pioglitazone does not induce hypoglycaemia episodes, it improves the lipid profile and has demonstrated certain cardiovascular benefits and a renoprotective effect in CKD patients.81 Its use is associated with sodium and water retention, oedema and an increased risk of heart failure,82 limiting its use in CKD patients; water-sodium retention is highest when it is used in combination with insulin. Furthermore, it increases the risk of osteoporosis and fractures, especially in post-menopausal women,83 and its extended use has been associated with a potential increase in bladder cancer.84
In conclusion, although pioglitazone can be used for any degree of CKD, its adverse effects (oedema, heart failure, fractures, risk of bladder carcinoma) limit its indication in patients with the aforementioned disease. The consensus recommends using it with caution in patients with a GFR <60ml/min/1.73m2 and avoiding its indication whenever the GFR is <30ml/min/1.73m2.
Alpha-glucosidase inhibitors
Both acarbose and miglitol and their metabolites accumulate in CKD, and as such, although it has not been documented that they increase the risk of hypoglycaemia, their use is not recommended,53 given their potential toxicity, especially to the liver,85 and their adverse gastrointestinal effects.
Dipeptidyl peptidase-4 inhibitors
There are currently 4 dipeptidyl peptidase-4 (DPP4) inhibitors sold in Spain: sitagliptin, vildagliptin, saxagliptin and linagliptin. The gliptins, by stimulating insulin secretion (which is dependent on glucose), have a very low risk of hypoglycaemia,77 and as such, their use is particularly attractive in CKD patients.86
Although they share the same action mechanism, gliptins have major pharmacokinetic differences that determine how they are used in the presence of CKD.
Sitagliptin, vildagliptin and saxagliptin are mainly eliminated via the kidneys, either without being metabolised (sitagliptin), or as active metabolites (vildagliptin and saxagliptin). As a result, these 3 drugs will require a dose adjustment when the GFR is <50ml/min/1.73m2. Sitagliptin should be used at doses of 50 and 25mg when the GFR is 50-30ml/min/1.73m2 and <30ml/min/1.73m2 (including dialysis), respectively.87 Vildagliptin should be used at doses of 50mg if the GFR is <50ml/min/1.73m2, including stage 5 CKD.88 Saxagliptin should be used at doses of 2.5mg in patients with a GFR <50ml/min/1.73m2; although saxagliptin is not indicated for use in patients with end-stage kidney disease or dialysis, a recent study has shown its safety in these cases.89
Linagliptin is eliminated through the hepatobiliary system, and therefore its half-life is hardly extended in CKD90. As such, a dose adjustment is not required, even in patients with advanced CKD,91 and it can also be used in diabetic dialysis patients.
In conclusion:
1. Gliptines are drugs whose efficacy and safety have been demonstrated in CKD patients.
2. They require a dose adjustment, with the exception of linagliptin.
3. Although they can be used in cases of advanced CKD or end-stage kidney disease, the experience of use in these patients is still limited.
Glucagon-like peptide-1 receptor agonists
The glucagon-like peptide-1 receptor agonists (GLP1-RA) currently sold in Spain are exenatide, exenatide LAR, lixisenatide and liraglutide. The first 3 derive from an animal protein, exendin, while liraglutide is a human GLP-1 analogue. Due to their duration, they can be classified as short-acting and long-acting GLP1-RA. The former include exenatide, which is administered twice a day, and lixisenatide, which is administered once a day; long-acting GLP1-RA include liraglutide, which is administered once a day, and exenatide LAR, which is administered once a week.
GLP1-RA, since they involve peptides, are eliminated by glomerular filtration, followed by tubular reabsorption and subsequent proteolytic degradation, which produces smaller peptides and amino acids that are reincorporated on protein metabolism. Although theoretically, due to not being metabolised by the liver or kidney or being eliminated in faeces or urine, they may be safe drugs without the need for dose adjustment in CKD patients, their use is limited by potential adverse effects and a lack of clinical experience in these cases.
According to their respective data sheets, exenatide, exenatide LAR and lixisenatide can be used without dose adjustment in patients with a GFR >50ml/min/1.73m2. Liraglutide does not require a dose adjustment with a GFR >60ml/min/1.73m2. Exenatide can be used in patients with a GFR between 30 and 50ml/min/1.73m2, with the dose being carefully increased, with a maximum dose of 5mg/12 hours. Lixisenatide, according to its data sheet, can also be used with caution in these cases.92 Although the efficacy and safety of liraglutide has been reported in patients with moderate CKD,93 the lack of clinical experience justifies the non-recommendation of its use in the data sheet when the GFR is <60ml/min/1.73m2. In patients with a GFR <30ml/min/1.73m2, GLP1-RA should not be used, given the lack of clinical experience.
GLP1-RA are the only anti-diabetic drugs that induce significant weight loss, and as such, they may be particularly indicated for patients with T2DM and obesity. Furthermore, they do not induce hypoglycaemia episodes, which is an advantage in patients with a high risk of suffering them, as occurs in CKD. Nevertheless, treatment with GLP1-RA is frequently associated with adverse gastrointestinal effects (nausea, vomiting, diarrhoea), which may be more common in CKD patients.93 As such, when we use GLP1-RA in CKD patients, it is important to monitor the patient’s tolerance and renal function in the event of vomiting or diarrhoea. The presence of neuropathy in the autonomous system and gastroparesis, common in patients with diabetes and CKD, could increase the occurrence of vomiting. GLP1-RA also induce natriuresis. Gastrointestinal and renal losses (especially in patients treated with diuretics or renin-angiotensin-aldosterone system inhibitors) may cause a contraction of extracellular fluid volume, which results in a deterioration in renal function in patients with previous renal dysfunction. Cases of acute renal failure have been described in patients treated with exenatide, both of pre-renal origin94 and due to acute interstitial nephritis.95
In conclusion:
1. There is little experience in the use of GLP1-RA in CKD patients.
2. The adverse gastrointestinal effects induced by GLP1-RA may be more common in patients with CKD.
3. Their use is currently limited to patients with mild-moderate CKD.
Sodium–glucose cotransporter 2 inhibitors
Sodium–glucose cotransporter 2 inhibitors (SGLT2-inh), such as dapagliflozin, canagliflozin and empagliflozin, act by inhibiting glucose reabsorption in the proximal tubule.
The efficacy of SGLT2-inh depends on renal function, and as such, it is reduced in patients with moderate CKD and is practically nil in advanced CKD.93
Dapagliflozin, the first drug of this family to be authorised in Spain, can be used without dose adjustment in patients with mild renal failure and it is not indicated in patients with a GFR <60ml/min/1.73m2. We recommend monitoring renal function before introducing dapagliflozin and at least annually (between 2 and 4 times a year in patients with impaired kidney function, and before starting concomitant treatment with medication that could reduce kidney function). If kidney function falls below a GFR of 60ml/min/1.73m2, treatment with dapagliflozin should be discontinued.94
These drugs are administered orally and do not cause hypoglycaemia; they induce weight loss and mild decreases in blood pressure. Their use is associated with an increase in urinary infections and genital mycosis. Given that they cause osmotic diuresis, mainly in severe hyperglycaemia, they can cause dehydration, high blood pressure and kidney function deterioration, especially in the elderly or in patients treated with antihypertensive drugs. Their concomitant use with diuretics is not recommended in situations of volume depletion (for example, acute gastroenteritis) or in individuals older than 75 years of age.
Insulin
CKD is associated with resistance to insulin. However, advanced CKD causes a decrease in renal insulin catabolism, and as such, a reduction in its dose is usually necessary, with it even being possible to discontinue insulin in some patients with T2DM and advanced CKD (burnt-out diabetes)95.
Insulin treatment in CKD patients requires strict monitoring in order that the aforementioned treatment may be adjusted; it is very important to ensure an appropriate therapeutic education in diabetes. The insulin therapy regimen will adapt to the control objectives and both conventional insulin treatment and intensive therapy may be considered.
As a general rule, which should be adapted to each patient on the basis of glycaemia monitoring, we can say that it is not necessary to adjust the insulin dose whenever the GFR does not fall below 60ml/min/1.73m2; below this GFR, the dose should be reduced by around 25%, and if it falls below 20ml/min/1.73m2 it should be reduced by 50%.96
Dialysis partially reverses the resistance to insulin and its lower catabolism associated with CKD. As a result, a patient’s insulin needs determined in a dialysis programme, will depend on the balance between the improvement of insulin sensitivity and the normalisation of insulin metabolism, and as such, treatment individualisation is essential.
It is recommended to monitor glycaemia in haemodialysis patients and bear in mind that after the session they are more susceptible to developing hypoglycaemia – the dialysate normally contains a glucose concentration of 100mg/dl, and as such, it may be advisable to decrease the insulin dose before dialysis and, in any case, be prepared in case of any post-dialysis hypoglycaemia episodes.97 It is recommended to use long-acting insulin (glargine, detemir or NPH) for basal requirements, with the addition of fast-acting insulin before meals, if necessary. Basal (glargine, detemir) and fast-acting analogues (aspart, lispro, glulisine) induce less hypoglycaemia than human insulins (NPH or regular), with the disadvantage of having a higher cost. In some patients, with very regular meal hours, pre-mixed insulins may be used. For patients with T2DM and stage 5 CKD, the total initial daily insulin dose is usually 0.25IU/kg, with subsequent individualised adjustments, in accordance with glycaemic self-control.98 The specific features of insulin treatment in dialysis patients are not included in the consensus’ targets. It must be highlighted that there are no fixed insulin therapy guidelines and that all clinical practice guidelines recommend individualisation and the support of an expert for diabetes cases that are difficult to control.
In peritoneal dialysis patients, the administration of intraperitoneal insulin may be preferable to its subcutaneous administration, since it achieves a better glycaemic control, although it worsens the lipid profile (decrease of high density lipoprotein cholesterol, increase of triglycerides).99 However, it is necessary to take into account that peritoneal dialysate has a very high glucose content, although the most recent intraperitoneal infusions have a lower amount or have replaced glucose with icodextrin. There are no fixed guidelines for these cases and, once again, individualisation is advisable.100
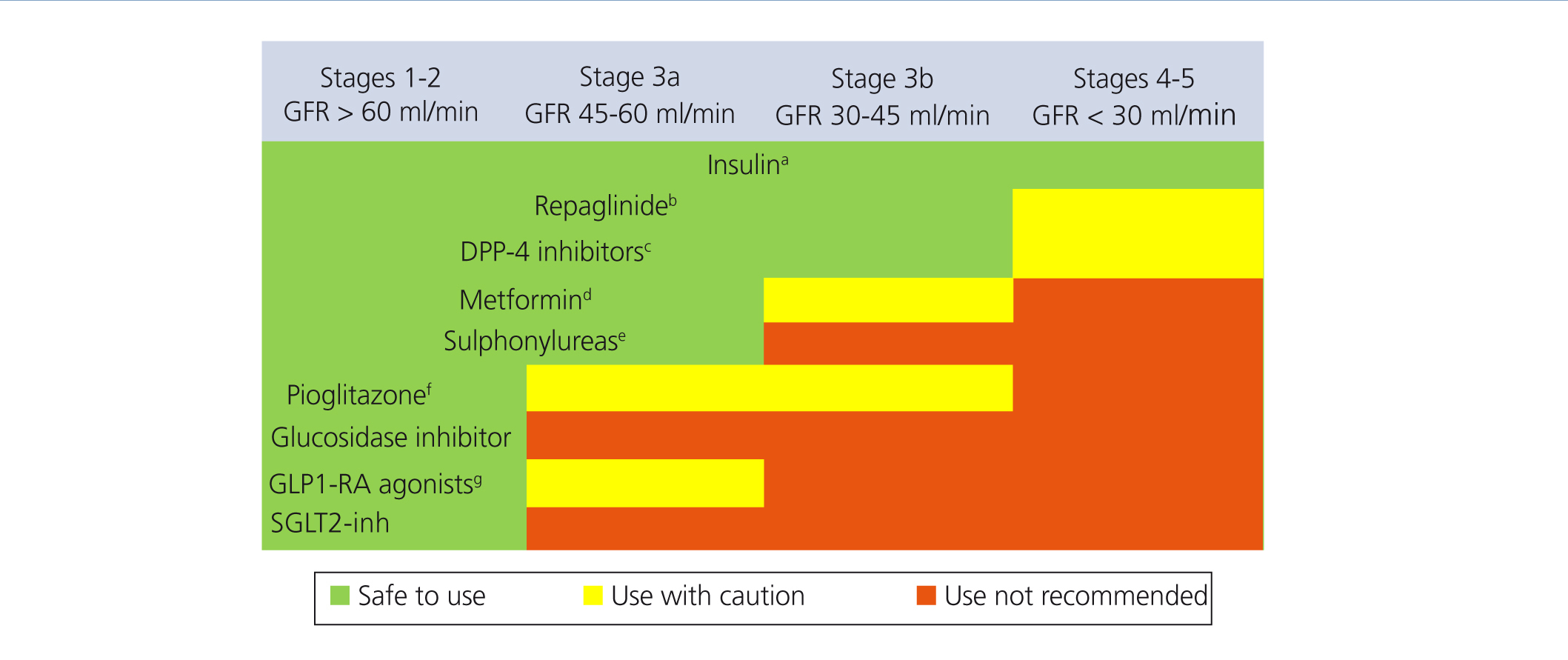
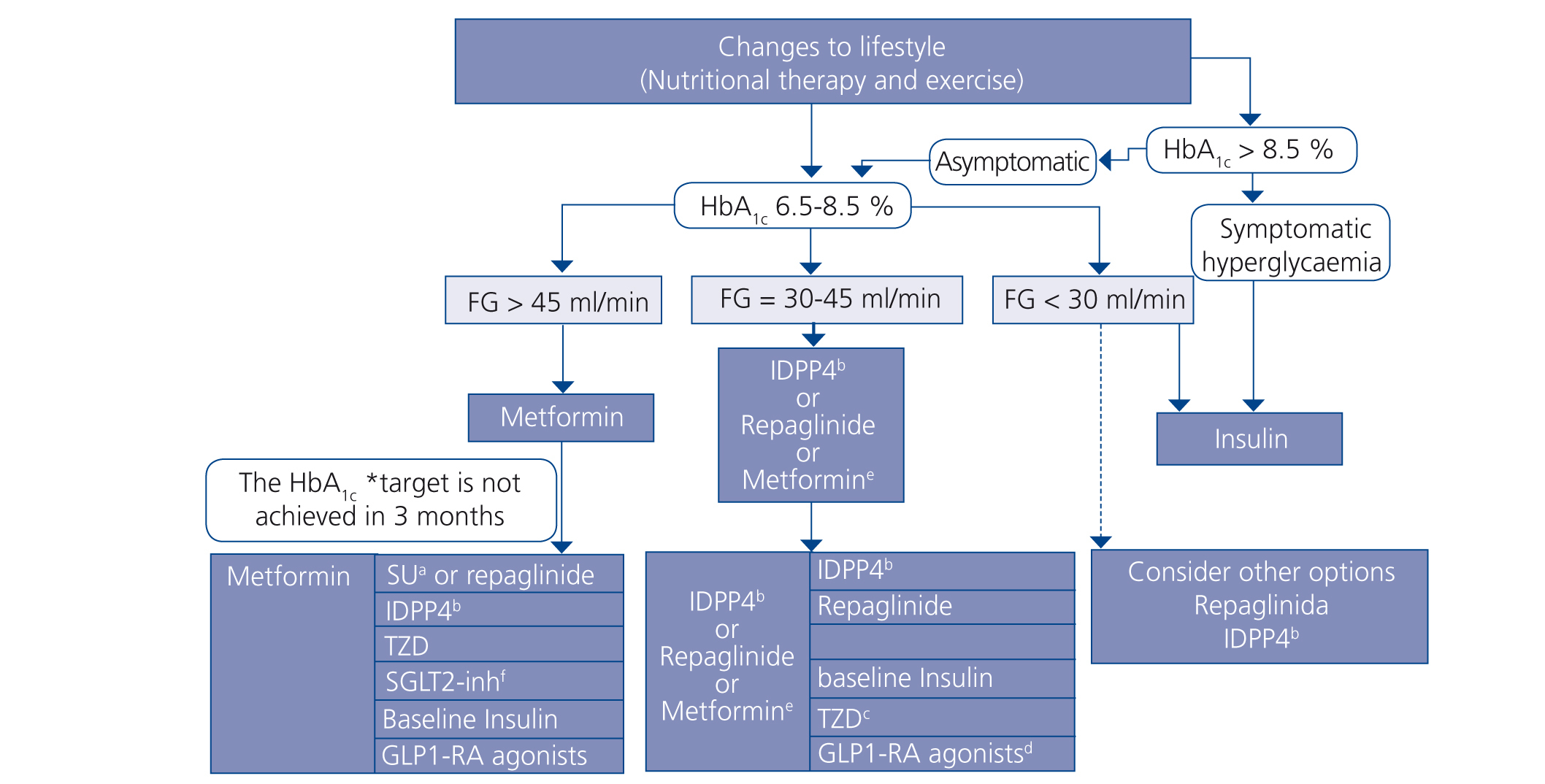
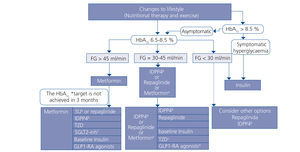
Choosing hypoglycaemic treatment in a chronic kidney disease patient (Figures 2 and 3)
Metformin continues to be the first line drug in the treatment of T2DM in all patients with an estimated GFR higher than 45ml/min/1.73m2.
When the GFR is between 30 and 45ml/min/1.73m2, metformin should be used prudently, given the risk of lactic acidosis, and it is recommended to reduce the dose and closely monitor kidney function. Both repaglinade and DPP4 inhibitors have demonstrated their use and safety in patients with this range of GFR values, although a reduction in the normal dose of these drugs is necessary, with the exception of linagliptin, which does not require a dose adjustment.
In patients with a GFR <30ml/min/1.73m2 or who are on dialysis, the experience with non-insulin anti-diabetic drugs has been very limited until present, and as such the treatment of choice should be insulin. However, in patients with not very marked hypoglycaemia, both repaglinade and DPP4 inhibitors are alternatives to be assessed.
In patients with a GFR >45ml/min/1.73m2 in whom the glycaemia control target has not been achieved with metformin, it can be combined, either with a DPP4 inhibitor or repaglinade, which are combinations with contrasting efficacy. If the control is still not adequate, basal insulin should be added. There is little experience with triple oral therapy in this population.
If the GFR is <45ml/min/1.73m2, the second step would be to combine DPP4 inhibitor with repaglinade and introduce basal insulin if the control target is not achieved. The combination of insulin with secretagogue drugs increases the risk of hypoglycaemia episodes, and as such, it is generally not recommended for these patients.
In conclusion, diabetes mellitus is a highly prevalent disease in CKD patients. We currently have many options for treating hypoglycaemia, which will continue to increase in the near future. The appropriate use of these drugs requires an extensive knowledge of their pharmacokinetics and safety profile by all professionals involved in treating the patient with diabetes and CKD.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Chronic kidney disease staging in accordance with the 2012 Kidney Disease Global Outcomes guidelines
Figure 2. Anti-diabetic drug indication in accordance with the degree of renal failure
Figure 3. Treatment algorithm in patients with type 2 diabetes mellitus and chronic kidney disease