La insuficiencia renal es una de las complicaciones más comunes e importantes en los receptores de trasplante hepático. Se ha descrito que ocurre con una incidencia del 17 % al 95 %. Esta complicación se asocia a una estancia prolongada en la unidad de cuidados intensivos, necesidad de diálisis posoperatoria, complicaciones infecciosas, rechazo agudo y aumento de la mortalidad. Las causas de deterioro de la función renal difieren entre los períodos pre y posoperatorio. Mediante la identificación de los pacientes con riesgo de desarrollo de una insuficiencia renal aguda y la implantación precoz de estrategias de protección renal es posible frenar la progresión de disfunción renal y mejorar los resultados a largo plazo de los receptores de trasplante hepático.
Renal failure is one of the most common and major complications in liver transplant recipients. It has been reported to occur at an incidence of 17% to 95%. This complication is associated with prolonged hospital stay in the intensive care unit, the need for postoperative dialysis, infectious complications, acute rejection, and increased mortality. The causes of renal function deterioration differ in the preoperative and postoperative periods. By identifying patients at risk of developing chronic renal failure and by implementing strategies for renal protection at an early stage, it is possible to slow down the progression of renal failure and improve the long-term outcomes in liver transplant recipients.
INTRODUCTION
The development of chronic renal failure (CRF) and chronic kidney disease (CKD) after an orthotopic liver transplantation (OLT) is associated with prolonged hospital stay1. The requirement for renal replacement therapy in the postoperative period, acute rejection and infectious complications lead to decreased survival. An analysis by the Scientific Registry of Transplant Recipients showed that CKD after non-renal organ transplantation was associated with a more than four times greater risk of mortality1. According to Charlton et al.2, post-transplant kidney injury, both acute and chronic, is associated with lower short- and long-term survival2.
Kidney injury is defined as the clinical and analytical deterioration of renal function if an increase in creatinine levels above 2mg/dl is detected, while an increase creatinine above 3mg/dl or 50% of the baseline value of already established renal dysfunction is considered to be renal failure. Oliguria may be present in both cases, with anuria being exclusive to renal failure3.
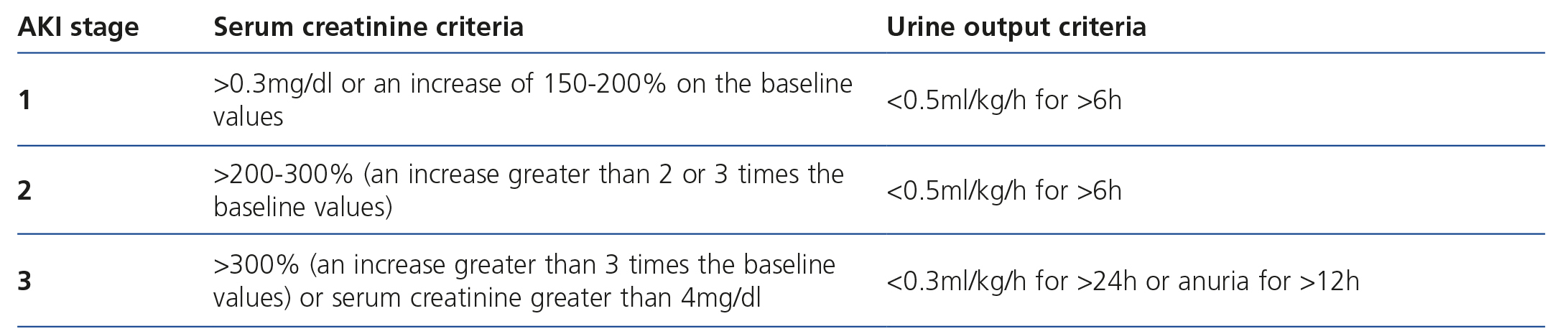
Classically, the RIFLE (Risk-Injury-Failure-Loss-Endstage) criteria were used to stratify risk in acute renal dysfunction, although it has been acknowledged that it had major limitations, and as such, in 20074,5, the Chronic renal failure Network (CRFN) revised these diagnostic criteria and proposed the classification of severity based on a modification of RIFLE criteria, which stratified the degree of renal dysfunction into five stages and reduced them to three stages in accordance with the increase in serum creatinine values and the decrease in cardiac and urine output4-6 (Table 1).
The estimated incidence of CRF following OLT ranges between 17% and 95%1. The main risk factors include hepatorenal syndrome (HRS), prolonged vena cava clamping time, low blood pressure in the intraoperative period and multiple transfusions.
The identification of risk factors2,7,8 and the development of renal protection strategies that minimise renal damage or its progression in patients with pre-existing CKD increase long-term survival and should be borne in mind as a priority in the management of OLT recipients.
The MELD (Model for end-stage Liver Disease) scale is a scoring system used to determine the severity of chronic liver disease, on the basis of which patients are prioritised on the OLT waiting list. This scale is useful for predicting survival of patients with liver disease and is calculated according to serum creatinine, bilirubin and the INR (International Normalized Ratio)9. Patients with high creatinine levels are considered a greater priority for OLT than those with normal renal function2,7,10,11. This has influenced a significant increase in the number of patients with renal dysfunction who receive transplants9. A comprehensive assessment of OLT candidates with renal dysfunction is required in order to determine who will benefit from a combined liver and kidney transplantation and in whom it is very likely that there will be a spontaneous recovery of renal dysfunction after transplantation10.
FACTORS ASSOCIATED WITH RENAL DYSFUNCTION IN THE PERIOPERATIVE PERIOD
CRF incidence following OLT is extremely variable, with values that range from 17%-95 %1,12, as a result of the disparity of criteria for defining this condition, with more than 35 different definitions having been published13,14. However, severe CRF, which is likely to require renal replacement therapy, has been documented in 5%-35% of cases1,15.
Post-OLT CRF has a multifactorial aetiology that is difficult to establish, and three different stages can be determined in relation to OLT: pre-transplant, intraoperative and post-transplant (Table 2). Below, we describe the risk factors associated with each and the strategies that may be employed for a management that optimises renal protection and decreases the incidence of kidney injury in OLT.
RISK FACTORS IN THE PRE-TRANSPLANT PERIOD
There is an association between various kidney disorders and liver disease; thus, for example, membranoproliferative glomerulonephritis is associated with α1-antitrypsin deficiency and hepatitis B and C, immune complex glomerulonephritis16 is associated with autoimmune hepatitis, and polycystic kidney disease is associated with polycystic liver disease3. Other causes of renal dysfunction include diabetes mellitus and hypertensive nephropathy4,17.
The traditional liver and kidney dysfunction model is pathophysiologically based on hyperaemia and splanchnic arterial vasodilation and increased cardiac output4,18 with compensatory activation of the renin-angiotensin-aldosterone axis13. This results in increased levels of catecholamine and angiotensin, causing intrarenal vasoconstriction and a decreased glomerular filtration rate (GFR), along with hyponatraemia. Recent studies have demonstrated the role of the renin-angiotensin-aldosterone system (RAAS) in the deterioration of liver function13 such that high levels of angiotensin are related to accelerated fibrosis development in animal research13. The progression of this situation leads to a situation of chronic renal failure and the resulting HRS3,19.
HRS is defined as the development of chronic renal failure in patients with advanced liver disease in the absence of an identifiable cause of renal failure20, which evolves to a clinical situation with major renal function deterioration, abnormalities in blood circulation and in the activity of endogenous vasoactive systems. All of this leads to renal vasoconstriction with a decrease in the GFR and arterial vasodilation with a decrease in peripheral vascular resistance and low blood pressure3. It is characterised by oliguria, hyponatraemia, hyperkalaemia, acid-base imbalance, increased serum urea, creatinine levels higher than 1.5mg/dl and creatinine clearance lower than 40ml/min, low levels of sodium in urine and increased osmolarity3.
HRS can be triggered spontaneously or as a result of infections, gastrointestinal bleeding, paracentesis or surgery3, and as such, all of these factors should be treated as early as possible to avoid its occurrence11.
HRS is classified according to its chronological course4, with there being two subtypes: type I, with rapidly and aggressively developing renal dysfunction, doubling of baseline serum creatinine values to 2.5mg/dl in less than two weeks and with mean survival of two weeks21,22. In type II, there is a more progressive deterioration of renal function, with a gradual increase23 in creatinine values above 1.5mg/dl and a 35% patient survival rate beyond one year4,21,24.
Type II HRS originates as a result of haemodynamic changes in the course of hepatic dysfunction, which may even precede the onset of ascites24. These haemodynamic changes include: splanchnic vasodilatation, reduction of effective blood volume, hyperdynamic circulation state with increased cardiac output, vasoconstriction of extra-splanchnic systems, including renal and cerebral circulation and increased RAAS activity. Type I HRS has a similar pathophysiology, but it occurs suddenly.
The treatment of choice while waiting for an OLT is vasoconstrictors such as terlipressin and ornipressin, the expansion of plasma volume4 or the insertion of transjugular intrahepatic portosystemic shunts that decrease portal hypertension, which are useful as a bridge to OLT3,24-26.
The identification of candidates for a combined liver and kidney transplantation is key, although very difficult. Recently published criteria27 include:
STRATEGIES FOR PREVENTING RISK FACTORS IN THE PRE-TRANSPLANT PERIOD
All necessary precautions must be taken to avoid the development of CRF or HRS. The potential benefits of diuretics, lactulose, exposure to iodinated contrasts, nephrotoxic drugs, non-steroidal anti-inflammatory drugs and selective cyclooxygenase 2 inhibitors must be carefully balanced with the risk of renal function deterioration, since they may accelerate a syndrome similar to SHR1,11,24,28-30.
Carrying out paracentesis with the extraction of large quantities of ascitic fluid in patients with hypoalbuminaemia and ascites without peripheral oedema increases the risk of excessive volume depletion and factors that accelerate CRF1, and as such, the need for it must be rigorously assessed. Paracentesis, by a mechanism that is not fully understood, causes a decrease in systemic vascular resistance and excessive activation of the RAAS1,31. The risk of post-paracentesis circulatory dysfunction decreases when plasma expanders are used, with human albumin being the treatment of choice32,33, which is more effective than other plasma expanders, although, it has not been related to increased survival20. In paracentesis with the extraction of less than 5l of ascitic fluid, the risk of post-paracentesis circulatory dysfunction is lower and, although colloids can be used, the international guidelines20 continue to recommend albumin as the treatment of choice, while, from 5l, the administration of 8g/l of extracted ascites is recommended1,20.
A recent meta-analysis by Salerno et al.34 confirms that albumin administration in spontaneous bacterial peritonitis (SBP) reduces the risk of kidney injury and mortality. Although the use of albumin in the doses reported by the authors is recommended in all patients with SBP20, the benefit of this treatment is greater in those with serum creatinine >1mg/dl, urea >30mg/dl or total bilirubin >4mg/dl20. Albumin use is only recommended in cases of SBP, and not in the presence of other infections.
In patients with SBP, the use of albumin infusions at doses of 1.5g/kg of body weight at the time of diagnosis, followed by 1g/kg three days later, has shown to reduce the risk of renal failure and mortality, mainly in patients with kidney injury and hyperbilirubinaemia1.
Given the high cost and high risk of bacterial resistance, the use of prophylactic antibiotics20 is controversial and is strictly restricted to patients with a high risk of SBP. Three populations have been identified in which their prophylactic administration is beneficial: patients with acute gastrointestinal bleeding, those with a protein count in ascitic fluid of less than 15g/l and those with previous episodes of SBP20.
In recent studies, it has been observed that biliary, gastrointestinal and urinary infections and SBP in patients with cirrhosis and ascites1 who have hyperbilirubinaemia result in a higher risk of CRF. The administration of albumin to prevent CRF when bilirubin values are higher than 4mg/dl is beneficial in these patients even with normal renal function parameters1.
RISK FACTORS IN THE INTRAOPERATIVE PERIOD
During the intraoperative period, there are often major haemodynamic changes and bleeding associated with different stages of OLT, that occasionally cause low blood pressure which may lead to renal hypoperfusion during transplantation3. Bleeding during OLT may occur as a result of a severe coagulopathy or in relation to the surgical techniques employed during liver dissection and in vascular reconstruction. Episodes of renal hypoperfusion have been reported that result from haemodynamic abnormalities associated with post-reperfusion syndrome3. There are various surgical techniques that maintain venous return in the anhepatic phase:
- Venovenous bypass.
- Preservation of the inferior vena cava (piggyback).
- Preservation of the inferior vena cava with maintenance of portal vein flow.
Clamping of the portal vein, the hepatic artery and the inferior vena cava during the anhepatic phase interrupts venous return to the lower limbs and to the splanchnic bed, resulting in a decrease in cardiac output, blood pressure, an increase in systemic vascular resistance and a reduction in vital organ perfusion, and could lead to renal hypoperfusion and potential ischaemic lesion1. Although carrying out a venovenous bypass has demonstrated greater haemodynamic stability, thus improving the venous return, it has not consistently been associated with a lower incidence of CRF in the immediate postoperative period1. The piggyback technique, with preservation of the inferior vena cava, results in fewer haemodynamic abnormalities than the previous techniques, thus improving venous return during the anhepatic phase, cardiac output and peripheral vascular resistance, and a lower incidence of CRF has been observed in the post-transplant period1,3 due to less retroperitoneal bleeding, since it is a technique that does not require retrocaval dissection1.
Other conditions related to the development of CRF during the immediate postoperative period include all non-OLT circumstances present in surgery1, such as the anaesthetic technique used, which may decrease effective blood volume, severe cardiovascular disease, cardiomyopathy, prolonged episodes of haemodynamic instability, low blood pressure, severe depletion of intravascular volume, the use of drugs that adversely affect intrarenal haemodynamics, advanced age, previous kidney disease and diabetes. An uncommon cause of CRF is obstructive tubulopathy due to pigments, including myoglobin, haemoglobin and bilirubin35,36.
Moreover, high blood transfusion requirements are associated with an increased incidence of CRF1.
RISK FACTORS IN THE IMMEDIATE POSTOPERATIVE PERIOD
The predisposing factors for CRF development in OLT recipients may be classified as:
- Drug toxicity.
- Other disorders related to the severity of the patient’s condition3 and allograft dysfunction29,37.
Nephrotoxic drugs are included in iodinated contrasts, antibiotics (mainly aminoglycosides, amphotericin B and aciclovir), treatment with immunosuppressants such as cyclosporine and tacrolimus, prolonged dopamine or vasopressor3 administration and multiple transfusions.
Other factors that may occur in liver transplant patients are similar to those of any other patient who remains in a critical care unit. We can include prolonged periods of low blood pressure, septic conditions, pre-renal kidney injury or clinical conditions typical of OLT, such as acute graft rejection or its primary dysfunction1.
STRATEGIES FOR REDUCING RISK FACTORS IN THE INTRAOPERATIVE AND IMMEDIATE POSTOPERATIVE PERIOD
In relation to the surgical technique
Certain surgical techniques previously discussed improve the haemodynamic state, mainly in the anhepatic phase, and have shown to be considerably beneficial against CRF development in the postoperative period (piggyback).
Plasma volume replacement and maintenance of renal perfusion
It is widely known that volume depletion is the most important risk factor for the development of post-transplant CRF38. There is controversy over most appropriate choice of fluid to resuscitate these patients. 0.9% NaCl or other potassium-free fluids are recommended in patients with renal dysfunction4. However, the use of large amounts of 0.9% NaCl is associated with hyperchloremic metabolic acidosis, which may lead to hyperkalaemia4. It is common for cirrhotic patients with a liver transplant to have varying degrees of hyponatraemia and its sudden correction with 0.9% NaCl increases the risk of post-transplant central pontine myelinolysis, and this factor must be borne in mind. Moreover, the administration of large volumes of potassium-containing solution, such as Lactated Ringer's (LR) solution, may cause hyperkalaemia in CRF patients.
In OLT, volume replacement is beneficial for maintaining mean arterial pressure (MAP) figures greater than 65mmHg, since lower figures are associated with renal hypoperfusion4. A hyperdynamic state due to vasodilation in liver failure may require the addition of noradrenaline to increase MAP and therefore optimise renal perfusion3.
There is controversy as regards the use of crystalloids or colloids as resuscitation fluids. Although the use of colloids will restore intravascular volume most effectively, there are studies in patients with OLT during their hospitalisation in the critical care unit that show a greater incidence of renal dysfunction and requirement for renal replacement therapies in patients resuscitated with colloids, with respect to those resuscitated with LR4.
Pharmacological interventions
Oliguria is determined by tubular obstruction with the accumulation of detritus during renal ischaemia. It is assumed that non-oliguric CRF has a better outcome and spontaneous recovery than oliguria4. Various drugs have been used to optimise renal function:
- Loop3 or osmotic diuretics: although early diuretic use improves diuresis and may transform oliguric CRF into non-oliguric CRF, a lower incidence of CRF or renal replacement therapy techniques has not been demonstrated after their use. They are recommended in cases of volume overload with cardiorespiratory compromise.
- Vasodilators that counteract renal vasoconstriction: dopamine, calcium channel blockers, prostaglandins and atrial natriuretic peptides have been used, amongst others, without positive results having been observed after their use3. In various meta-analyses4, it has been demonstrated that dopamine does not prevent the development of CRF in patients with OLT4, and is associated with increased mortality and arrhythmogenic events in studies carried out on septic shock that compare dopamine with noradrenaline4. Fenoldopam is a selective dopamine-1 receptor agonist that may prevent the development of CRF, although there are mixed results with regard to this premise4.
- Immunosuppressants: calcineurin inhibitors are the most nephrotoxic immunosuppressants and as such, several strategies have been developed in an attempt to preserve renal function, which include the use of low doses and/or the delay in their introduction via combination with anti-interleukin-2 (IL-2) receptor antibodies and/or mycophenolate (MMF). If there is previous kidney injury, we recommend delaying treatment with calcineurin inhibitors (cyclosporine and tacrolimus) by 3 to 7 days1, and instead using anti-lymphocyte antibodies or MMF. Whenever they are introduced, blood levels must be monitored and it must be borne in mind that they can be altered by the use of other drugs, such as various antimicrobial agents3. There are currently new non-nephrotoxic immunosuppressant agents with immunosuppressant action demonstrated, such as IL-2 receptor antagonists, mTOR (target of rapamycin molecule) inhibitors, sirolimus and everolimus10,39, or antilymphocyte preparations that may delay or replace the administration of calcineurin inhibitors in patients at risk of developing CRF or with previous renal function deterioration1. The risk of graft rejection must be assessed before this is determined1.
In general, mTOR inhibitors are not used during the first three months after transplantation, since they worsen scarring and increase the risk of hepatic artery thrombosis. Numerous studies39 have shown that early use of these strategies (during the first year after transplantation), such as the administration of mTOR inhibitors with low doses of calcineurin inhibitors or their total withdrawal, significantly improves medium-long term renal function, with a low incidence of rejection.
Anti-thymocyte globulin is practically restricted to steroid-resistant acute rejection in the context of liver transplantation and is not administered as a general rule, since its use has only been approved by the Food and Drug Administration in renal transplantation and aplastic anaemia.
MMF is not nephrotoxic, but it may be less effective as an immunosuppressant, with a higher risk of late graft rejection11.
Renal replacement therapies
In various multicentre studies4 where fluid balance in patients with CRF has been analysed, it has been observed that this balance was more positive in non-surviving patient groups with oliguric CRF or those who required renal replacement therapy4. The requirement for pre-transplant dialysis may be associated with higher mortality than the MELD scale19. Narayanan Menon et al.40 found mortality to be five times higher in this patient subgroup.
Current results indicate that early renal replacement therapy after OLT improves survival in CRF patients. We require randomised clinical trials that confirm this hypothesis, which is currently based on prospective studies4.
Currently, renal replacement techniques allow ultrafiltration to be carried out with preferential fluid loss if urea and creatinine levels are moderately high, continuous haemodiafiltration if there is greater metabolic involvement or conventional haemodialysis. Continuous techniques are preferred, due to their better haemodynamic tolerance, lower increase in intracranial pressure and better control of circulating volume, although the risk of bleeding is greater, given the permanent anti-clotting of the circuit4. These patients are likely to be treated with conventional anti-clotting agents by heparinisation of the circuit, although a small number are resistant to this treatment and require alternatives such as anti-clotting with citrates or prostacyclins.
New biomarkers for the early detection of renal dysfunction
Although renal function deterioration in severe liver disease has been related to haemodynamic abnormalities that compromise renal blood flow, in recent studies, it has been discovered that the intestines play an important role13 as mediators between the kidneys and liver, with high values of interleukin 17A (IL-17A) being secreted by intestinal Paneth cells13, which is related to renal dysfunction mainly during the reperfusion phase13,41.
Classically, the increase in serum creatinine levels has been used for the diagnosis of renal dysfunction, but it is a highly insensitive marker of renal function deterioration13,17. Creatinine may be lower as a result of low muscle mass (which is very common in cirrhotic patients) or an increase in the GFR due to drugs that are often prescribed in these patients19. As such, the diagnosis of CRF based on plasma urea is complicated due to the great variability in its levels, since high levels may be found in episodes of digestive bleeding or in situations with increased protein catabolism, relative hypovolaemia and HRS, amongst others, and lower levels may be found due to insufficient synthesis in severe liver disease.
Many more sensitive and earlier biomarkers than increased creatinine in liver disease42 have been developed to predict renal function deterioration13. One of those most studied is called serum or urinary9 neutrophil gelatinase-associated lipocalin (NGAL)13,42, a 25 KDa protein whose levels rapidly change in renal dysfunction. It can be determined both in the pre- and post-transplant period, and it is considered to be the earliest marker of renal failure, allowing strategies that prevent major complications to be established. Although more studies are required to corroborate the above13,43-45, it is a promising biomarker in this context42.
Cystatin C is a peptide produced by nucleated cells and it is an ideal marker of renal dysfunction in cirrhosis of the liver. Its values are not changed by muscle mass, diet or inflammatory conditions. However, it is an expensive method that is not universally available19.
Numerous biomarkers are currently being researched.
In conclusion, renal dysfunction in the context of OLT is associated with longer hospital stay46,47, higher costs46-48, post-transplant sepsis and mortality. We must determine CRF development risk factors in the pre-transplant period, the intraoperative period and the immediate postoperative period, and develop renal protection strategies that minimise renal damage or its progression in order to improve long-term survival. These strategies should be carried out as a priority in patients with OLT who develop CRF or in those with pre-existing renal function deterioration, in an attempt to avoid its progression, and we recommend a multidisciplinary approach that includes all professionals involved in the OLT process.
KEY CONCEPTS
- The development of ARF in liver transplant recipients is one of the most common problems (with an incidence of 17-95%) and which increases hospital stay most.
- Various strategies for renal protection can be carried out during the pre-transplant, intra-operatory and post-operatory phase, with the aim of preserving renal function.
- A pre-operative exhaustive evaluation is recommended to determine which patients will benefit from hepatorenal transplant and in which patients a spontaneous recovery of renal function after OLT is predicted.
- Renal replacement therapy prior to OLT is associated with an increase in mortality five times greater in the post-operatory period.
- Episodes of bleeding, infections, hypotension and hypovolaemia in the pre-operatory period are closely related with ARF development and must be treated aggressively.
- The new biomarkers predict deterioration of renal function early on, providing promising NGAL results.
- Calcineurin inhibitors used as immunosuppressive treatment in the post-transplant period play a significant role in the development of renal failure due to their considerable nephrotoxicity.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Current CRF stage classification of the Acute Kidney Injury Network (CRFN)(4).