Osteoporosis (OP) and chronic kidney disease (CKD) independently influence bone and cardiovascular health. A considerable number of patients with CKD, especially those with stages 3a to 5D, have a significantly reduced bone mineral density leading to a high risk of fracture and a significant increase in associated morbidity and mortality. Independently of classic OP related to age and/or gender, the mechanical properties of bone are also affected by inherent risk factors for CKD (“uraemic OP”). In the first part of this review, we will analyse the general concepts regarding bone mineral density, OP and fractures, which have been largely undervalued until now by nephrologists due to the lack of evidence and diagnostic difficulties in the context of CKD. It has now been proven that a reduced bone mineral density is highly predictive of fracture risk in CKD patients, although it does not allow a distinction to be made between the causes which generate it (hyperparathyroidism, adynamic bone disease and/or senile osteoporosis, etc.). Therefore, in the second part, we will analyse the therapeutic indications in different CKD stages. In any case, the individual assessment of factors which represent a higher or lower risk of fracture, the quantification of this risk (i.e. using tools such as FRAX®) and the potential indications for densitometry in patients with CKD could represent an important first step pending new clinical guidelines based on randomised studies which do not exclude CKD patients, all the while avoiding therapeutic nihilism in an area of growing importance.
Osteoporosis (OP) y enfermedad renal crónica (ERC) influyen de manera independiente en la salud ósea y cardiovascular. Un número significativo de pacientes con ERC, especialmente desde estadios 3a a 5D, presentan una disminución significativa de la densidad mineral ósea condicionando un alto riesgo de fractura y un incremento importante de la morbimortalidad asociada. Independientemente de la OP clásica asociada a edad y/o sexo, las propiedades mecánicas del hueso se encuentran afectadas adicionalmente por factores intrínsecos a la ERC («OP urémica»). En la primera parte de esta revisión, analizaremos conceptos generales sobre densidad mineral ósea, OP y fracturas, en gran parte infravalorados hasta ahora por los nefrólogos debido a la falta de evidencias y a las dificultades diagnósticas en el contexto de la ERC. Actualmente se ha demostrado que una densidad mineral ósea disminuida es realmente predictiva del riesgo de fracturas en pacientes con ERC, aunque no permite distinguir entre las causas que la originan (hiperparatiroidismo, enfermedad adinámica del hueso y/o osteoporosis senil, etc.). Por ello, en la segunda parte analizaremos las implicaciones terapéuticas en distintos estadios de la ERC. En cualquier caso, la valoración individualizada de los factores mayores y menores del riesgo de fractura, la cuantificación de dicho riesgo (i.e. con el uso de herramientas como el FRAX®) y las indicaciones potenciales de densitometría en pacientes con ERC podrían constituir un primer paso importante en espera de nuevas guías clínicas basadas en estudios aleatorizados que no excluyan a pacientes con ERC, evitando mientras tanto nihilismo terapéutico en un área de creciente importancia.
Osteoporosis (OP) is the most common skeletal disorder in the general population,1 and is characterised by a loss of bone strength associated with an increased risk of low-impact fractures and their negative consequences.2–4 Bone strength is determined not only by bone mineral density (BMD), but also by bone quality2–4 (Fig. 1). However, OP is usually diagnosed on the basis of BMD below a predetermined and arbitrary level (T-score≤−2.5 standard deviations) measured by “Dual-energy X-ray Absorptiometry” (DEXA), without taking into account bone quality.2,5 It is important to emphasise that the BMD value is an important risk factor for fracture, and that a large proportion of fractures in the general population occur in individuals with osteopenia,6 so it is also essential to assess other non-densitometric risk factors (Table 1).7–10
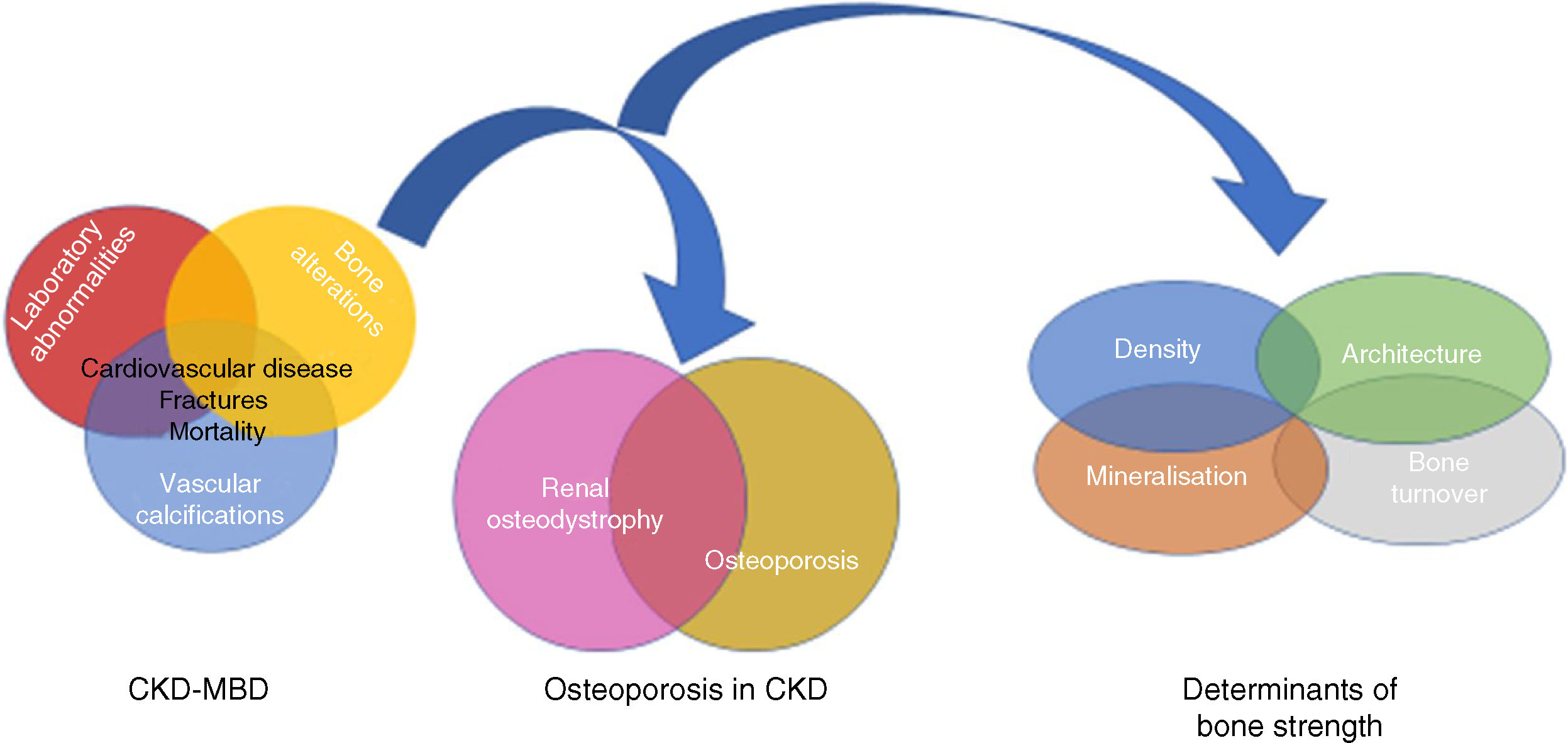
Relationship between Chronic Kidney Disease–Mineral and Bone Disorder (CKD–MBD), renal osteodystrophy (bone changes secondary to chronic kidney disease [CKD]) and OP (associated with uraemia or age and gender of patients, among other factors). Bone strength is determined not only by bone mineral density, but also by bone quality, expressed by its determinants.94,151 Although some authors use the term “uraemic” OP,17 it is important to remember the existence of non-terminal CKD, which could be integrated within the CKD–MBD complex due to its capacity to worsen the condition.
Fracture risk factors.
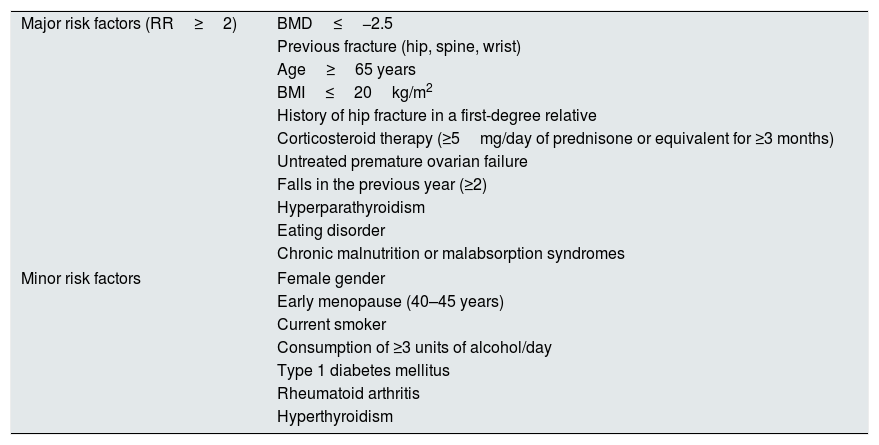
| Major risk factors (RR≥2) | BMD≤−2.5 |
| Previous fracture (hip, spine, wrist) | |
| Age≥65 years | |
| BMI≤20kg/m2 | |
| History of hip fracture in a first-degree relative | |
| Corticosteroid therapy (≥5mg/day of prednisone or equivalent for ≥3 months) | |
| Untreated premature ovarian failure | |
| Falls in the previous year (≥2) | |
| Hyperparathyroidism | |
| Eating disorder | |
| Chronic malnutrition or malabsorption syndromes | |
| Minor risk factors | Female gender |
| Early menopause (40–45 years) | |
| Current smoker | |
| Consumption of ≥3 units of alcohol/day | |
| Type 1 diabetes mellitus | |
| Rheumatoid arthritis | |
| Hyperthyroidism | |
BMD measured as T-score (number of standard deviations from BMD of women aged 20–29) exponentially increases the risk of fracture.146 Osteopenia (T between −1 and −2.5): doubles the risk of fracture (2×); osteoporosis (T≤−2.5): 4×; established osteoporosis (T≤−2.5 and fracture); severe osteoporosis (T<−3.5). The Z index (i.e. ≤−2) should be used for diagnostic purposes in the assessment of BMD in pre-menopausal women and men under 50 years of age.101 The Z value indicates the relationship with the “expected” value for the patient's age. In the absence of BMD measurement, this could be indicated by the presence of a major risk factor (other than age) or 2 minor risk factors, or, according to different guidelines, 2 major or 1 major+2 minor. Other risk factors important for nephrologists would be (among others, and by different mechanisms): the use of loop diuretics, chronic use of heparin or anticoagulants, proton pump inhibitors, antihistamines, selective serotonin reuptake inhibitors, oestrogen and testosterone blockers, antiepileptics, aromatase inhibitors, etc.147–150 As fractures occur at a younger age in CKD, SEN 2011 Spanish guidelines suggested that, in addition to transplant patients, densitometry should be performed in women over 50 years of age and men >65 years of age with CKD (unlike the usual indication in women >65 years of age and men >70 years of age).101
BMD: bone mineral density; BMI: body mass index; RR: relative risk.
On the other hand, chronic kidney disease (CKD) is known to have an important impact on bone health, as defined in the classical concept of “renal osteodystrophy” (ROD).11,12 Currently, the term ROD should be used exclusively to define histological lesions observed in bone biopsies in patients with CKD (one of the components of the “Chronic Kidney Disease–Mineral and Bone Disorder” (CKD–MBD) complex,13 which includes Turnover, Mineralization and Volume -TMV- abnormalities).8,11,12 ROD includes diseases with high bone remodelling, such as osteitis fibrosa (reflecting secondary hyperparathyroidism), low bone remodelling (such as osteomalacia or adynamic bone disease [ABD]), and mixed forms, among others. OP, meanwhile, involves a loss of bone mass and changes in microarchitecture not associated with a specific mineralisation, cellularity or bone turnover defect.14,15 Therefore, although OP and ABD share some common clinical characteristics, their pathogenesis, histopathology and treatment are different.14,16
There is a fast-growing body of evidence that patients with CKD have a higher risk of fracture (and associated mortality) than the general population, probably because the mechanical properties of the bone are additionally affected by intrinsic “uremic factors” specific to CKD. This has led to the introduction of a new concept “uraemic OP”,17 which emphasises the particularly complex relationship between BMD and the risk of fracture and mortality in CKD patients, since this population is also exposed to “classic” age- or gender-related OP even before the diagnosis of CKD.17 For this reason, nephrologists must, on the one hand, gain further insight into the risk factors for OP and fracture, and, on the other, promote the diagnostic criteria of the classic form of OP. We should also highlight the importance of CKD in the differential diagnosis of patients with OP, in light of the therapeutic implications that will be analysed in the second part of this review.18 In fact, secondary hyperparathyroidism characteristic of CKD is most probably not the primary cause of fractures,17,19 and loss of BMD associated with population ageing, sex hormone changes and other secondary causes should be considered, irrespective of the loss associated with CKD or ROD itself.17,19–21
For all these reasons, in the first part of this review we will analyse in detail both the evaluation of fracture risk in the general population and in CKD patients, and the possible indications of DEXA, given its diagnostic implications in CKD. Biochemical parameters (biomarkers) and the use (infrequent and sometimes limiting) of bone biopsy will also be analysed.
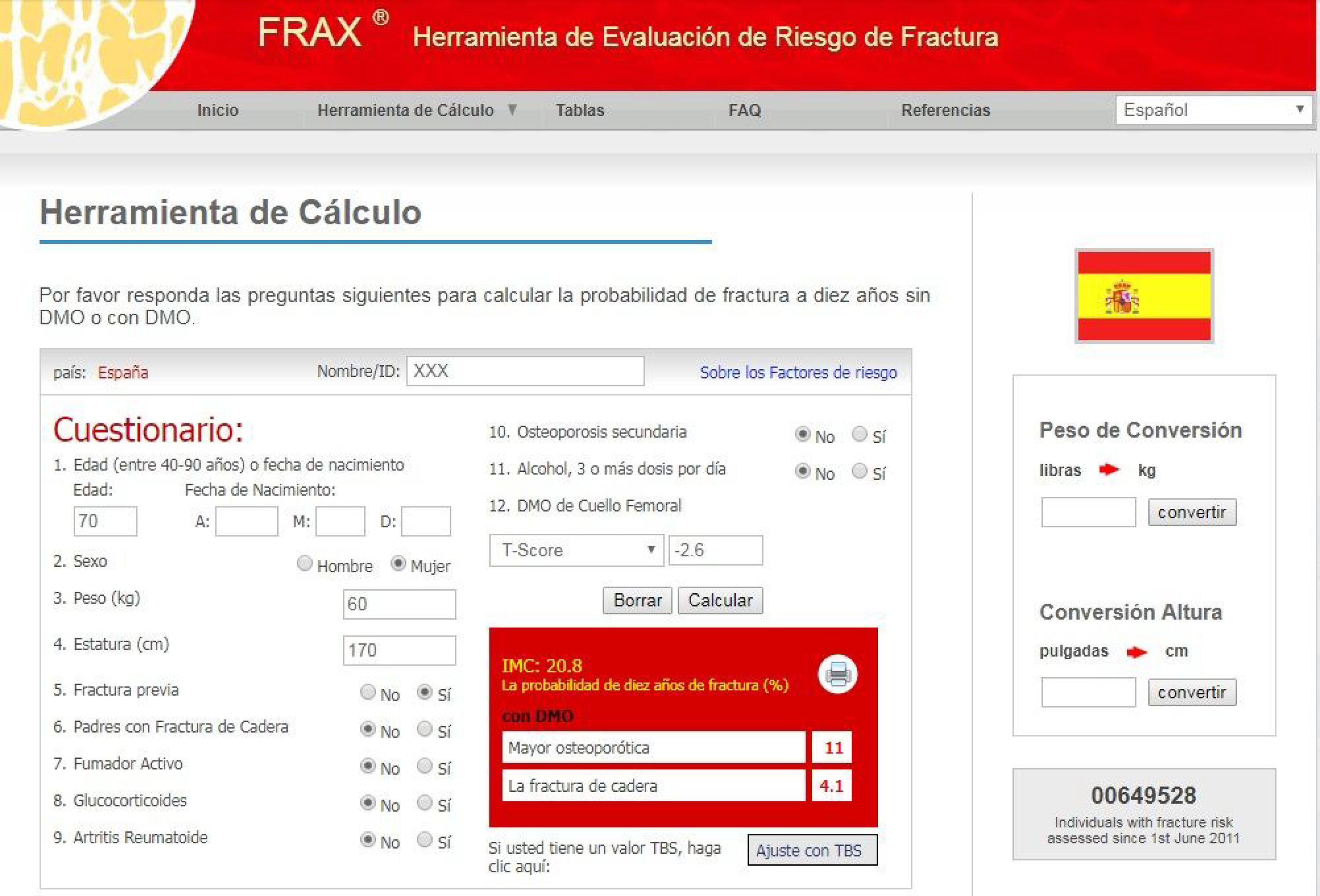
General concepts of osteoporosis, risk factors and clinical consequencesOsteoporotic or fragility fractures (spontaneous or caused by minimal trauma [such as a fall from the same height]) are a significant public health problem due to their high prevalence, morbidity and mortality, and increasing consumption of resources.22–25 For this reason, various agencies25 recommend evaluating the presence of fracture risk factors (Table 1) on an individual basis, and discourage universal densitometry screening.3,26–29 Risk factors should be evaluated whenever there is clinical suspicion, and perhaps also, ideally, in all patients with CKD, regardless of age. On the other hand, quantification of fracture risk in the general population can be performed using different scales, the best known being the Fracture Risk Assessment Tool (FRAX®) (www.shef.ac.uk/FRAX) (Fig. 2). The FRAX® algorithm calculates the 10-year probability of major osteoporotic fracture (vertebral, forearm, hip or humerus) and/or hip fracture (without current or previous treatment). The scale has been translated and validated in various countries, and the score is merely illustrative, given its significant limitations (Fig. 2).10,30
Example of the Fracture Risk Assessment Tool (FRAX®): for Spain (http://www.shef.ac.uk/FRAX/tool.aspx?country=4). The FRAX® algorithm calculates the probability of a major osteoporotic fracture in a specific country. In addition to the obvious factors shown, previous or current administration of corticosteroids for more than 3 months (5mg or more of prednisolone or equivalent), OP concomitant with rheumatoid arthritis, OP secondary to disorders closely linked to it (type 1 diabetes, adult osteogenesis imperfecta, chronic untreated hyperthyroidism, hypogonadism or premature menopause, chronic malnutrition, malabsorption and chronic liver disease), ingestion of more than three units of alcohol per day, and finally, optionally, BMD at the neck of the femur are all taken into consideration. When entering BMD values in the table, the trabecular bone score, if available, can also be entered later. The shortcomings of FRAX® include the use of dichotomous variables (yes/no), and the absence of certain variables, such as the number of previous fractures, the corticosteroid dose and the number of falls suffered. In addition, it does not differentiate between vertebral and non-vertebral fractures, the evaluation of secondary OP is incomplete (kidney disease or glomerular filtration is not taken into account, among other causes), and concerns have been raised about the representativeness of the Spanish cohort.46,47,115 In centres where DEXA is not available for BMD measurement, FRAX® may be particularly useful in selecting patients for referral for DEXA.
The FRAX® algorithm does not include CKD, suggesting that this scale will underestimate the risk of fracture in our patients, especially in those with advanced CKD.31,32 Indeed, it is striking that a recently published set of American guidelines only include terminal CKD as a cause or contributor to OP and fractures.33 In any event, although FRAX® does not include adjustments for estimated glomerular filtration rate (eGFR), we believe that nephrologists could use this tool in a preliminary assessment, considering that the absolute value obtained probably underestimates the real risk of fracture. In fact, the presence of CKD is not only an important independent risk factor for fracture, but also increases the frequency of falls due to muscular weakness – sarcopenia or myoneuropathy.34–40 FRAX® has been shown to discriminate and predict fractures in CKD or kidney transplant patients.41–43 For example, Jamal et al.,41 reported that the discriminative capacity of BMD at the femoral neck was similar to FRAX® for morphometric vertebral fractures and any fracture, with FRAX® being superior for clinical non-vertebral fractures (0.66; [0.60–0.73]). Compared with FRAX®, the area under the curve for age was lower in all types of fracture, but the best results were observed with FRAX®+BMD.41 Similarly, Naylor et al.42 analysed 320 patients (67±10 years, 71% women) with eGFR<60ml/min/1.73m2 and 1787 with eGFR≥60ml/min/1.73m2. The observed risk of major clinical fracture due to OP was 5.3% (3.3–8.6%) in patients with eGFR<60ml/min/1.73m2 (comparable to the FRAX® estimate [6.4% with BMD and 8.2% without BMD]). No significant differences in prediction were observed in individuals with eGFR > or <60ml/min/1.73m2. In this study, FRAX®+BMD, FRAX® without BMD, and femoral neck BMD predicted fractures with an area under the curve of 0.65–0.71.42 FRAX® has also been assessed as a predictor of fracture in kidney transplant patients43 and, recently, as a predictor of mortality in Japanese patients on dialysis.44 Despite these positive findings, additional studies are needed before FRAX® can be recommended in routine practice, particularly in stages 4–5D, since the presence of major changes in mineral metabolism (i.e. severe secondary hyperparathyroidism) or its treatment (vitamin D, phosphate binders) in these stages may be significant enough to affect the accuracy or adequacy of both FRAX® and the criteria used for the diagnosis, prognosis or treatment of OP.
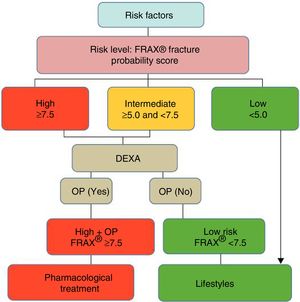
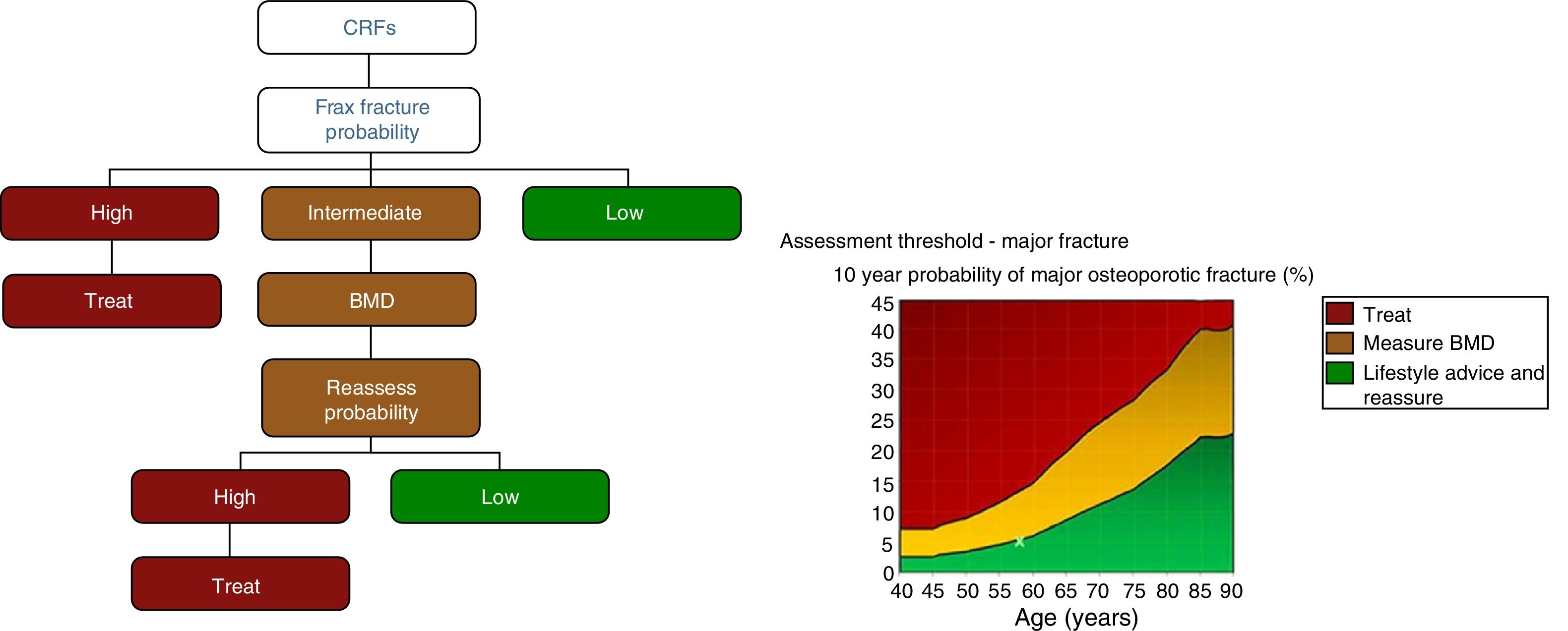
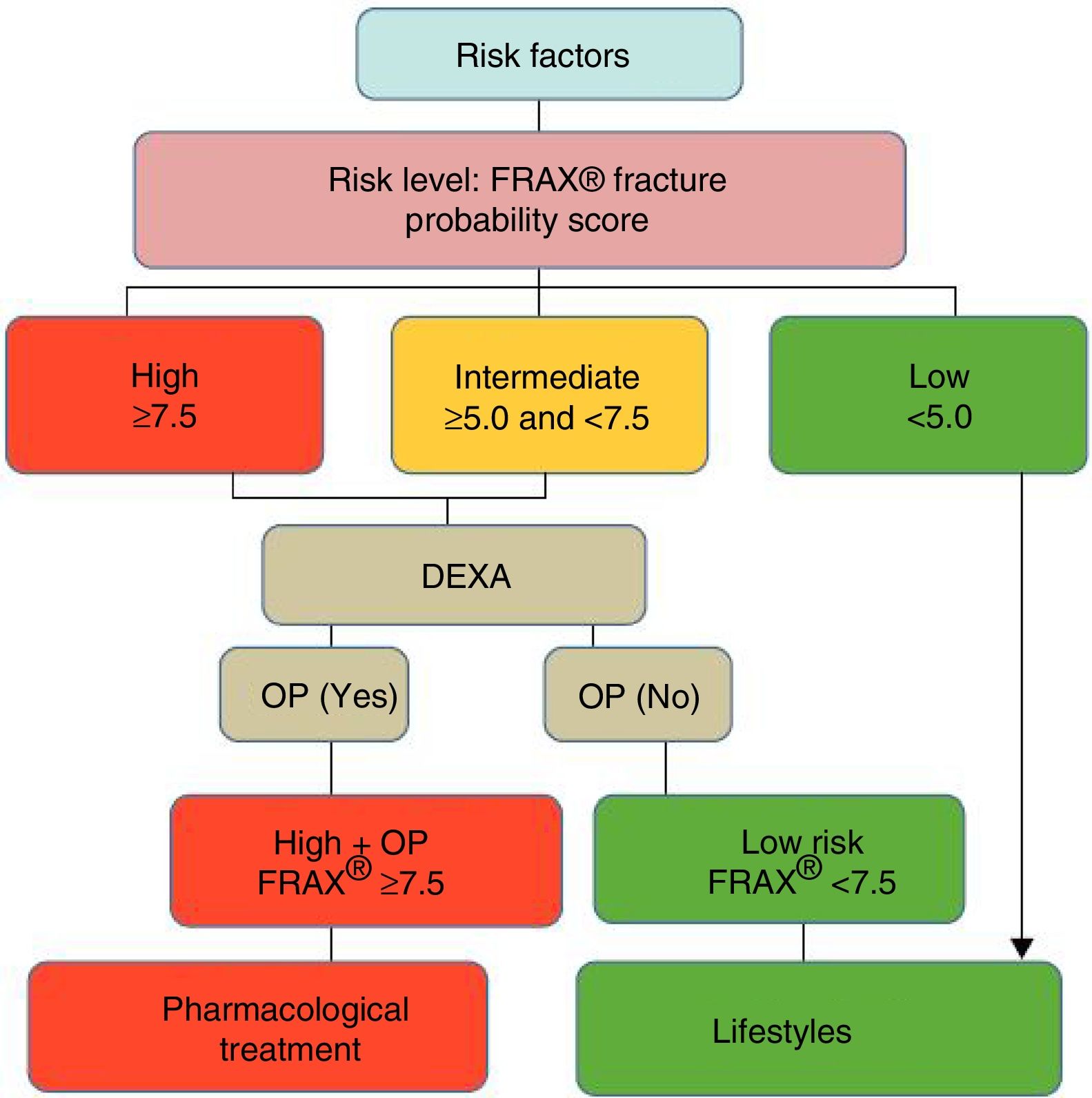
After quantifying the fracture risk with FRAX® (in the absence of a BMD measurement), patients are classified as low, intermediate or high risk (Fig. 3). Low risk patients should simply receive general advice (diet, exercise and re-evaluation at 5 years). Intermediate risk patients, depending on the country and resources available, are usually evaluated by densitometry to recalculate FRAX® (including in this case BMD data). In the general population, active therapeutic intervention is advised in patients with high-risk FRAX® and those whose re-evaluated risk falls above a certain threshold (i.e. >10% for major fractures and/or >3% for hip fracture, according to countries and authors) (Fig. 3). Given that FRAX® appears to underestimate the risk of fracture in the Spanish cohort,45 later studies have re-evaluated its usefulness in our general population,46,47 and densitometry/treatment is now advised in patients with >7.5% 10-year probability of major osteoporotic fracture (Fig. 4).
Estimation of fracture probability using FRAX® (fracture probability according to risk factors [CRF]). High, intermediate, and low risk levels vary according to guidelines and countries. In the United States and the United Kingdom, ≥20% 10-year probability of major osteoporotic fracture or ≥3% probability of hip fracture is considered high, 10–20% is considered an intermediate probability (densitometry [BMD, bone mineral density] and recalculation is recommended), and <10% is considered a low probability (figures based on cost-effectiveness). Some guidelines (see Fig. 4) recommend recalculating FRAX (reassess probability) using BMD for both intermediate- and high-risk patients before treating (BMD can also be used to evaluate response to treatment).26.
Decision tree based on the most cost-effective option in the Spanish FRIDEX cohort of Spanish women (general population) who did not receive treatment during the 10 years of follow-up.47 DXA or DEXA: Dual-Energy X-ray absorptiometry; OP: osteoporosis.
Finally, we believe it is important to point out that an increasing number of studies have recognised the close link between vascular disease and bone pathology.48 There is a significant inverse relationship between cardiovascular morbidity and mortality and BMD in both the general population and in CKD patients,49,50 and a similar inverse relationship between BMD and vascular calcification.48,51–54 The paradox of vascular calcification in the context of bone decalcification has been described in different pathologies.55 Other studies have described the association of vascular calcification with a higher prevalence of vertebral fractures.56,57 Moreover, the prognostic value of these calcifications has been demonstrated using a simple lateral X-ray of the lumbar spine (Kauppila index) or of the hands and pelvis (Adragao index).8,58 Therefore, extending the lateral lumbar X-ray (suggested for the evaluation of aortic calcifications) to the thoracic region could be useful in the detection of asymptomatic vertebral fractures (Table 1).
Epidemiology of fractures in chronic kidney diseaseDialysis or transplant patients with CKD stages 1–5 have a much higher risk of suffering a fracture at any age compared with individuals of the same age and gender.24,37,59–62 Aside from the known risk of OP in patients with renal transplantation or treated with corticosteroids, there is increasing evidence of a reduction in both BMD and the mechanical properties of bone in patients with CKD stages 3a–5D.63–68 In a Canadian cohort of 679,114 adults aged ≥40, the cumulative incidence of peripheral and axial fractures increased gradually and significantly in adults in parallel with a decrease in eGFR in both genders and age groups (40–65/>65 years).37 In stage 5, up to 10% of women and 5% of men experienced at least 1 fracture after 3 years of follow-up, with a similar trend in falls requiring hospitalisation.37 In another recent study (n=10,955), both eGFR and albuminuria were significant risk factors for fracture.69 All these data may be underestimated, since risk increases even in patients with relatively preserved renal function if cystatin C is used as a surrogate marker of renal function.70,71 Furthermore, the risk of fracture seems to increase even after acute deterioration of renal function that requires dialysis, despite almost complete recovery of renal function.72 Finally, it should be noted that several longitudinal studies have confirmed the existence of an independent relationship between impaired renal function and accelerated BMD loss with age.35,73–76
In dialysis patients, several studies have also shown an increase in the incidence of fractures, especially of the hip.64–68,77–80 In an international cohort (n=34,579), Tentori et al. reported that 3% of participants presented with a fracture, although this incidence varied considerably among different countries (12/1000 patients-year in Japan; 40/1000 patients-year in Belgium and Sweden).64 In this study, Spain presented the second lowest global incidence of fractures, although the incidence of hip fractures was similar to that of other European countries, suggesting that other fractures (such as vertebral) could have been underestimated. In any event, fractures were more frequent in the group of dialysis patients vs. the general population in all countries64, and non-vertebral fractures were always much more frequent than vertebral fractures.59–62 Age, female gender, hypoalbuminaemia, previous kidney transplantation, diabetes, cardiovascular disease or dementia have been found to be predictive factors,77,78,81 as well as taking selective serotonin reuptake inhibitors, narcotics and opiates, benzodiazepines, antiepileptic drugs and, of course, corticosteroids.21,77,78,81 Finally, a Danish study that collected data on almost all types of fracture79,82 found the risk to be 3 times higher in patients on dialysis (twice as high in transplant patients) compared to healthy subjects.79 These figures are lower than those reported in previous studies.65–68,83 It is important to note that all these fractures occur at a younger age (approximately 10 years younger) and are associated with a significant increase in morbidity and mortality.64 For example, mortality (unadjusted) is 3.7 times higher and the death/rehospitalisation rate is 4 times higher in patients on dialysis with fractures compared with patients with no fractures.42,63,64,77,78,84
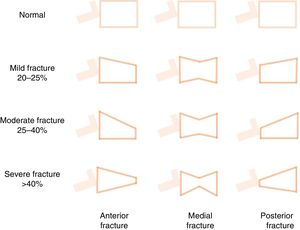
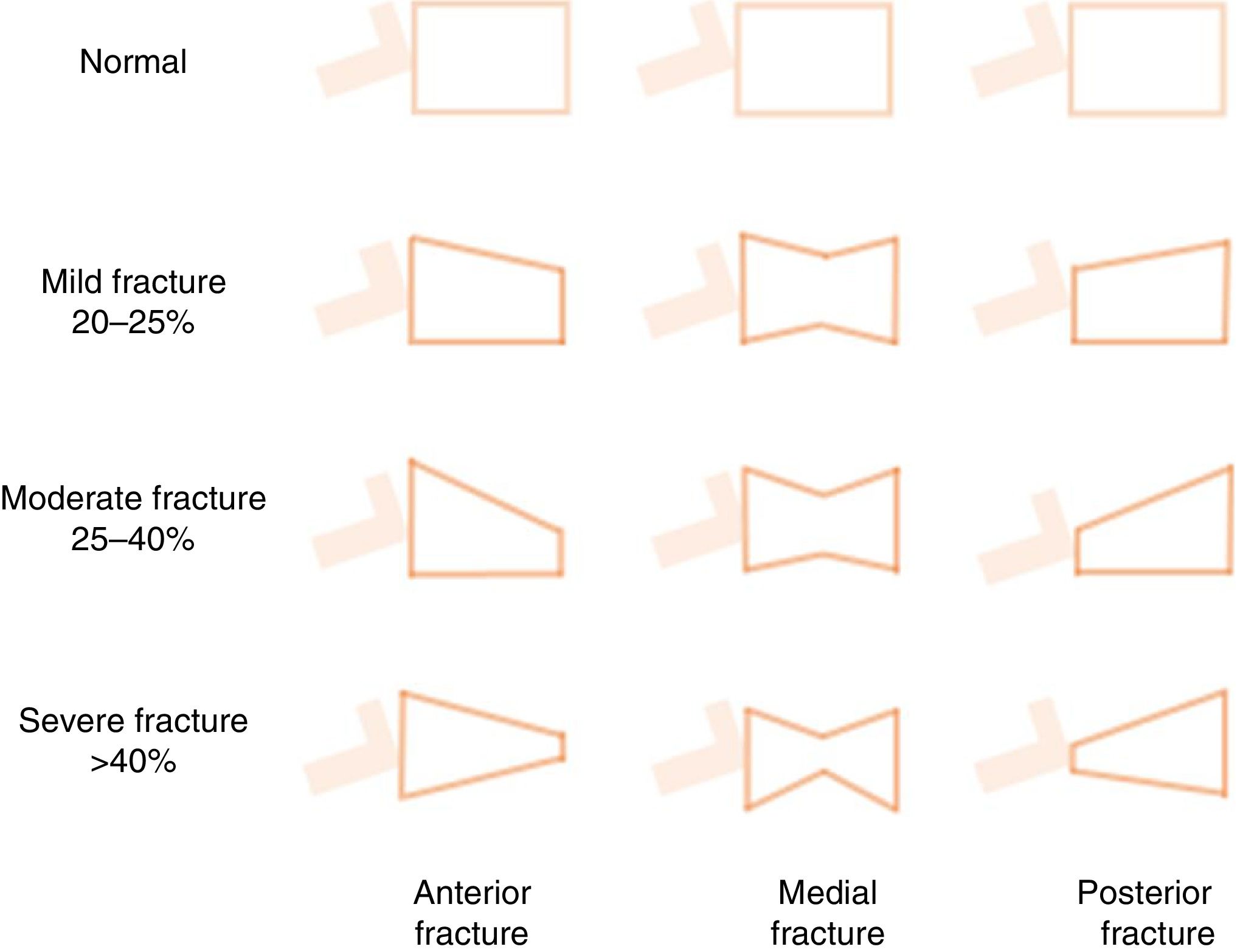
Vertebral fractures are the most common outcome of OP, and are also a major risk factor for other fractures and morbidity and mortality.3,56,82,85,86 They are frequently misdiagnosed as acute lower back pain, while in other cases they can be silent, insidious and progressive, and are only diagnosed fortuitously by the foreshortening of the vertebral body (morphometric vertebral fracture) (Fig. 5). More than 2/3 of vertebral fractures remain undiagnosed in the general population86 and are found in more than 25% of patients undergoing pre-transplant workup.87 In addition, the combined use of BMD and detection of vertebral fractures appears to improve the assessment of vital risk. For example, Genant's classification (Fig. 5),85 though rarely used by nephrologists,88,89 has proven useful in the general population, and seems to have prognostic utility in patients with CKD89 or on dialysis.88 Currently, vertebral morphometry (“Vertebral Fracture Assessment” or “Lateral Vertebral Acquisition”) can be performed on lateral dorsal-lumbar column images using a densitometer90; the technique has also been used in patients with CKD.91 Finally, it is important to note that the risk of vertebral fracture does not appear to be clearly higher in patients with CKD56 or in those at different stages of pre-dialysis CKD.92 This may be due to the different mechanical properties of bone elasticity and the forces applied (vertical or parallel) to different types of bone (cortical vs. trabecular) in these patients.17 Rodríguez-García et al., in a Spanish population of 193 patients on dialysis, found the prevalence of vertebral fractures to be 26.5% vs. 24.1% in the general population.56 The risk of hip fracture in CKD, however, is clearly higher than in the general population,17 being 3- to 4-fold higher in dialysis patients vs. the general population and patients without dialysis.78,79 This, of course, is the most serious consequence of OP, since it is associated with an increased risk of new fractures and premature death, and underscores the importance of performing interventions to reduce this risk.14,56,64,90,93
Schematic representation of Genant's semi-quantitative approach to the visual measurement of vertebral deformities. Normal=0; mild=1; moderate=2; severe=3; doubtful=0.5. Vertebral fractures are often diagnosed fortuitously (morphometric fracture), although diagnosis can also be made on the basis of symptoms. It is based on more than 20% loss of vertebral body height in any of the vertebral segments. The following formula is used: ([posterior height of the vertebral body−lowest height]/posterior height)×100.115,152
In routine clinical practice, diagnosis of OP is based on the measurement of BMD by DEXA.2,14,94 Though still the “gold standard”, the accuracy of this scanning technique varies greatly, and does not take bone quality into account.95 Despite the differential characteristics of bone fragility in patients with CKD, measurement of BMD should probably follow the same indications as for the general population, especially in patients with intermediate or high risk of fractures (Table 1, Figs. 2–4).27 Obviously, as mentioned in current guidelines, BMD should be performed only when its result can impact therapeutic decisions.96
The measurement of BMD by DEXA, or less frequently by computed tomography (CT), is also a useful tool for the evaluation of bone fragility in patients with CKD.27,97 However, the relationship between bone fracture and BMD in these patients is more complex. Thus, certain anatomical and histological features must be taken into account when interpreting the results in patients with CKD, since cortical bone involvement is more prevalent in these patients.94,98 DEXA cannot distinguish between these features, and relies on location to indicate more cortical (radius, femur) or trabecular (lumbar) involvement. Furthermore, DEXA can overestimate BMD in the spine, particularly in patients with CKD, due to the increase in aortic calcifications and the high prevalence of lumbar osteoarthritis.14,27
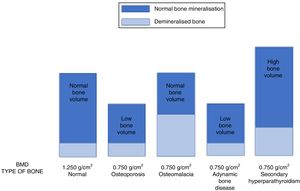
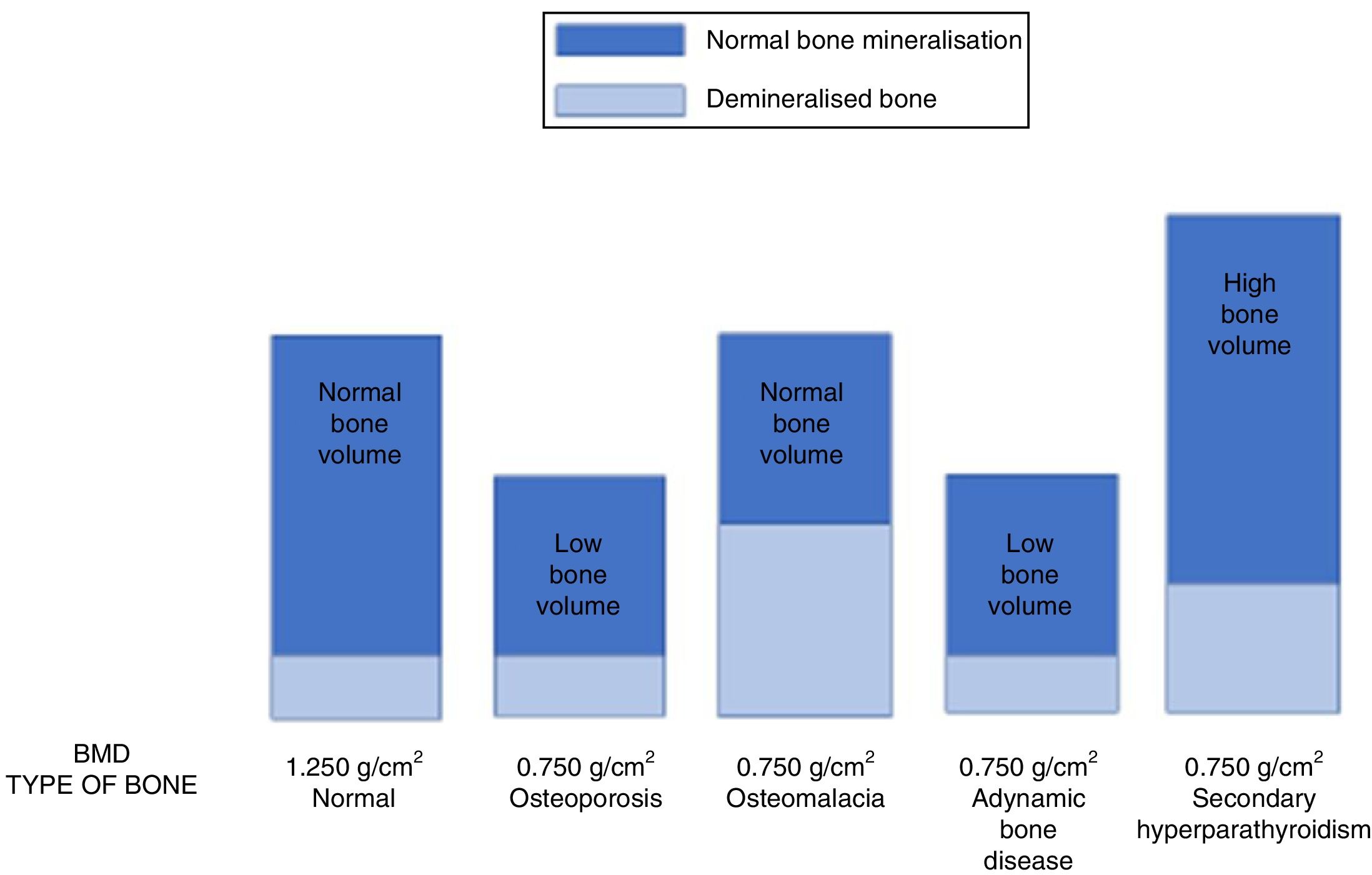
It is very important to consider that the different forms of ROD can show a similar decrease in BMD in CKD patients.8 Therefore, using DEXA, patients with high-turnover or low-turnover ROD can show the same densitometric measurements as a classic “senile” OP profile (Fig. 6). This is why the 2009 KDIGO guidelines13 established that “in patients with CKD stages 3–5D with evidence of CKD–MBD, we suggest that BMD testing not be performed routinely, because BMD does not predict fracture risk as it does in the general population, and BMD does not predict the type of ROD”.13 In fact, this suggestion was based on the assumption that loss of BMD could essentially be due to the calcium-phosphorous metabolism abnormalities associated with CKD (e.g. hyperparathyroidism), and that controlling phosphorus and parathyroid hormone levels was considered to be safer and more appropriate for the control of ROD than antiresorptive therapy, especially in patients with eGFR<30ml/min/1.73m2.13,96 On the other hand, a diagnosis of “OP” in an individual, without considering the possible coexistence of CKD, would require a different clinical approach involving the use of antiresorptive agents that could lead to the onset or worsening of ABD.13,99
The image shows how different pathologies (senile osteoporosis or osteoporosis secondary to hypogonadism, osteomalacia, adynamic bone disease and secondary hyperparathyroidism) can show the same low bone mineral density (in this example, BMD=0.750g/cm2) although they are caused by a completely different bone composition, and require different treatment strategies.112,133
However, previous observations showing that decreased BMD is more common in patients with CKD stages 3–4 have now been confirmed, and, as mentioned above, several longitudinal studies have also confirmed the existence of an independent relationship between changes in renal function and accelerated loss of BMD with age.35,73–76 Furthermore, there has been no solid evidence of the relationship between BMD and risk of fracture in patients on dialysis, with studies showing various associations or no association at all.27 However, a recent meta-analysis and systematic review of 13 published studies on the potential association between DEXA and fractures in patients with CKD (pre-dialysis and dialysis)100 showed that BMD was significantly lower in the femoral neck, lumbar spine, distal third of radius and ultradistal radius in patients with fractures, irrespective of dialysis. Although this meta-analysis has obvious limitations, it did suggest that BMD is able to predict the risk of fracture in patients with CKD,100 while in patients with hyperparathyroidism, the distal 1/3 of the radius could be more representative of changes in cortical bone, as recommended in the 2015 International Society for Clinical Densitometry guidelines.101 Several studies show that this location is predictive of the risk of fracture in patients with CKD 3–5D,101–103 but should not be measured in the arm carrying the AV fistula.104 In addition, at least 5 prospective cohort studies evaluating DEXA and incidence of fractures in adults with CKD stages 3a–5D have confirmed the good predictive capacity of BMD in patients with CKD.42,102,105–107
The first such study was performed in 485 Japanese patients on haemodialysis, and showed that baseline BMD (femoral neck and total hip) was independently associated with an increase in the incidence of any type of fracture (HR 0.65 [95% CI=0.47–0.90]).105 Other authors102 have shown that BMD measured by DEXA (total hip, lumbar spine, ultra-distal and distal third of radius) and high-resolution peripheral quantitative CT scan (HR-pQCT) of the radius could predict fractures in pre-dialysis adults with stage 3–5 CKD.102 Yencheck et al., meanwhile, evaluated the association between BMD by DEXA and fractures in a prospective study in 2,754 non-institutionalised older individuals (mean age 73.6 years),106 confirming the association between decreased BMD in the femoral neck and a higher risk of fracture, with or without CKD.106 Finally, in a recent study of 1,426 individuals aged ≥40 (mean 67 years), Naylor et al.,107 showed that, irrespective of BMD, individuals with CKD and a trabecular bone score (TBS) from lumbar spine DEXA below the median (<1.277) were 3 times more likely to suffer a fracture at 5 years. TBS is a texture analysis obtained by DEXA imaging that correlates with bone microarchitecture.108,109 The authors also showed that the association between TBS and fracture was independent of BMD and other risk factors. However, this sample included a limited number of CKD patients (especially advanced stage), so further validation is needed.24,31 Similar findings have been described in transplant patients.110
As mentioned above, quantitative CT can distinguish between cortical and trabecular bone. Studies using quantitative CT, for example, have found that lumbar cortical BMD is the best predictor of vertebral fractures in dialysis patients.111 Quantitative CT identified prospectively more bone loss at the hip than DEXA.97 There is also evidence that reduction of cortical BMD in the radius measured by CT increased the risk of fracture 16 fold35 and, more recently, that HR-pQCT allowed visualisation of ultrastructural details that could improve its predictive value.31,63,112–114 However, this scanning technique was not superior to DEXA in other cohorts.102 HR-pQCT does not, of course, provide the information on bone turnover and mineralisation that can be obtained from bone biopsy, and it is expensive and not widely available.63
Other tests that are more portable and therefore used for mass screening are peripheral DEXA (forearm, finger or heel) and quantitative ultrasound (QUS) bone densitometry, usually in the calcaneus. Their results are more limited and not equivalent to DEXA; moreover, these techniques are less precise and accurate, and abnormal results should be confirmed with central DEXA.33 Other non-invasive techniques, such as magnetic resonance and different spectroscopic methods, have also been used in a research context to evaluate bone quality.63 One of the most interesting techniques is bone microindentation, which involves delivering a microscopic impact that directly measures the mechanical strength of bone.115–118
Biochemical parameters and risk of fractureA detailed review of the utility of bone remodelling biomarkers in the diagnosis and therapeutic management of ROD and/or patients with OP is beyond the scope of this review, so we refer the reader to the general guidelines and other recently published reviews.8,13,27,96,119 Nevertheless, it is important to bear in mind that in the absence of bone biopsy, and despite various controversies, intact PTH and/or bone alkaline phosphatase levels are the best (albeit suboptimal) surrogate biomarkers for CKD histology studies.112 Intact PTH (inverted U- or J-shaped curve) and alkaline phosphatase (linear relationship) are also predictors of survival in these patients.120,121 The lowest mortality risk is observed in patients on dialysis who have PTH values between 150 and 300pg/ml (2× to 5× the upper limit of normal),120,122,123 or approximately 400pg/ml, according to a recent study.124
There is some consensus that the sensitivity and specificity of PTH levels in patients on dialysis is greater in the low range, where it is associated with ABD (i.e. PTH levels less than 2× the lower limit of normal),16 or in the very high range (9× the upper limit of normal), where it is associated with osteitis fibrosa secondary to hyperparathyroidism. Both low and high levels of PTH have been associated with a decrease in BMD and a high incidence of fractures.77,83,125–127 For instance, Atsumi et al. reported that Japanese patients with PTH in the lower tertile had a 2.4-fold higher risk of vertebral fracture than those in the medium tertile, and a 1.6-fold higher risk than those in the top tertile.127 In the DOPPS study, in contrast, levels >900pg/ml were associated with the highest prevalence of fractures.128 Differences in the strength of association between BMD-risk of fracture and other parameters is evidenced by the results of another study, in which the risk was less severe in patients with PTH >65pg/ml than in those with PTH <65pg/ml.106 In addition, in another sample of Japanese patients on haemodialysis, it was observed that PTH levels both lower and higher than the standard 150–300pg/ml were associated with incident fractures (HR 3.47 and 5.88, respectively).105 In the same study, elevated bone alkaline phosphatase was also associated with incident fractures.
On the other hand, there are no clear data to suggest that biomarkers (such as moderate increases in PTH) are associated with loss of bone strength or increased incidence of fractures in patients with stage 1–3 and possibly stage 4 CKD. Therefore, in the absence of clear, persistent metabolic abnormalities, the first cause of fracture in these patients could be “classic” OP,94 for which new therapeutic interventions are available.24,63,96
There is little information about plasma vitamin D levels (calcidiol [25OH-vitamin D]), bone histology, and risk of fracture in patients with CKD.127 Low levels of calcidiol (<20ng/ml) have been correlated with bone turnover, the rate of osteoid synthesis and mineralisation, and static histomorphometric parameters in dialysis patients.129 In other populations with CKD, both receiving and not receiving dialysis, evidence of a correlation between levels of vitamin D and lumbar or radial BMD has been reported40,130–133; however, other studies found no such association.134 In the study by Ambrus et al., both decreased levels of calcidiol and low levels of PTH, among others, were independent predictors of fracture risk.132 For all these reasons, determination of calcidiol levels could guide replacement therapy in these patients.18,135,136
Other biomarkers (propeptides, telopeptides, etc.) are, generally speaking, of little use in patients with CKD and/or do not correlate with predicted bone loss or response to treatment,9 and their use has scant benefit in daily clinical practice.27 Most biomarkers are excreted in urine,94 and can therefore be elevated in CKD independently, while others are significantly influenced by haemodialysis.137 Some recent publications, pending confirmation, show a potential predictive utility for some new markers, such as FGF-23 or sclerostin.97,138–141 FGF23 could be a marker of bone mineralisation (inversely related to osteoid accumulation)142 by regulating non-tissue-specific alkaline phosphatase, independently of Klotho, through the FGFR3 receptor,140 and elevated levels of FGF23 found in CKD (negatively associated with BMD)143 could contribute to bone loss by stimulating Dkk1 through a Klotho-mediated process.144 Aside from this, it is clear that all these biomarkers are still of little use in the context of CKD and/or OP.
Bone biopsyDouble tetracycline labelling is still the “gold standard” method of evaluating bone turnover and other dimensions of ROD,112,145,146 although it is rarely used due to the logistical difficulties involved. However, there is no evidence to date of a correlation between fractures, type of ROD or histomorphometric variables,27,63 and prospective studies to compare DEXA, HR-pQCT and histomorphometry are needed.63 New perspectives on the evaluation of cortical bone and immunohistochemical techniques could revalidate the need for bone biopsies in nephrology and rheumatology,145 and could help distinguish between OP and “classic” forms of ROD (especially ABD). For example, recent studies have reported that femoral BMD is associated with cortical porosity,146 and a higher stage of kidney disease is associated with thinner cortices, which could contribute to higher risk of fracture in this population.147 Although there is no irrefutable evidence that antiresorptive agents cause ABD,96 or that their administration in a patient with ABD can affect bone strength, until the publication of the new guidelines,96 it seemed reasonable to exclude ABD before starting this therapy, particularly in patients with eGFR <30ml/min/1.73m2.13,14,94
In contrast, some authors have ventured to suggest that the introduction of the concept of “uraemic OP” challenges the consensus of bone biopsy as the gold standard diagnostic technique and heralds a paradigm shift.17,24 Those in favour of this change argue that bone formation and mineralization rates may not be the most determinant factors of fracture propensity, because the relationship between these factors and bone chemical properties remains unknown.17 We have already mentioned that it is unclear whether changes in mineral metabolism are major determinants of fracture risk in patients with CKD,82 that the role of PTH levels (at both ends of the scale) is controversial or marginal, and that no correlation has yet been observed between calcium and phosphorus levels and risk of fracture.83,125 These data suggest that strategies to prevent fractures in patients with CKD must take into account other factors that are also found in the general population and are not solely related to the traditional aims of our intervention.3,24,82 From a practical point of view, on the other hand, the 2009 KDIGO guidelines recommended performing a bone biopsy before starting antiresorptive treatment in patients with eGFR <30ml/min/1.73m2, but the logistical difficulties involved (performance of the biopsy and external diagnosis) could prevent patients with CKD from receiving the treatment they need.24 These factors and their therapeutic repercussions will be discussed in the second part of this review.18
ConclusionPatients with CKD have a higher risk of bone fractures than the general population, with non-vertebral fractures being even more common than vertebral fractures. Given the association between fractures and increased morbidity and mortality, we believe that nephrologists should evaluate other risk factors, and should quantify the risk of fracture (especially in patients with mild-moderate CKD) using methods and tools similar to those used in the general population (i.e. FRAX®, BMD). In fact, the predictive capacity of these techniques, even in the presence of CKD, has been demonstrated in several studies. For this reason, the new KDIGO 2017 guidelines suggest using BMD to assess the risk of fracture in patients with CKD 3a–5D with evidence of CKD–MBD and/or OP risk factors if the results can impact therapeutic decisions (evidence 2B).96 This could involve additional interventions to reduce falls, and the administration of drugs to treat OP in the case of low or progressively decreasing BMD. Therefore, we believe that at least in selected groups of patients with factors associated with increased risk of fracture, with no severe biochemical changes and/or serial determinations (i.e. 2-yearly) that show frank bone loss,101 treatment of OP should be considered individually and therapeutic nihilism should be avoided. The availability of bone biopsy should not always be a limiting factor.24,96 Finally, we encourage nephrologists to pay close attention to the latest information in this relatively new area, and call on researchers to conduct prospective studies that do not systematically exclude patients with CKD.
Key concepts- •
OP and CKD independently influence bone and cardiovascular health.
- •
Patients with CKD may also present “classic” OP, such as that associated with age and/or gender.
- •
A significant number of patients with CKD present significant loss of BMD.
- •
Loss of BMD determines not only a high risk of fracture but also a significant increase in associated morbidity and mortality.
- •
There is evidence that loss of BMD is also predictive of fracture risk in patients with CKD, although it could underestimate the risk of fracture, particularly in stages 4–5D.
- •
BMD alone does not distinguish between its underlying causes (hyperparathyroidism, adynamic bone disease and/or senile osteoporosis, etc.).
- •
In patients with CKD (especially mild-moderate), risk factors for fracture should be assessed and quantified if possible (i.e. with FRAX®) in a similar way to the general population.
- •
The 2017 KDIGO guidelines suggest using BMD to assess the risk of fracture in patients with CKD with evidence of CKD–MBD and/or OP risk factors, if the results can impact therapeutic decisions.
- •
The possibility of treating OP, with or without a prior bone biopsy, should at least be considered in selected groups of patients and individually (i.e. with factors associated with a high risk of fracture and in the absence of severe, persistent biochemical alterations).
- •
New guidelines steer clinicians away from therapeutic nihilism due to the recognised importance of fractures and their complications in CKD.
The authors declare that they have no conflicts of interest.
This review includes authors who belong to the Red Nacional RedinRen [National Kidney Research Network] (RD06/0016/0001 and RD12/0021/0033), the Red de Biobancos Nacional Española [Spanish National Biobank Network] (RD09/0076/00064) and the Grupo Catalán de Investigación [Catalan Research Group AGAUR] (2009 SGR-1116), as well as contributors from the Fundación Iñigo Álvarez de Toledo [Iñigo Álvarez de Toledo Foundation] (FRIAT). We also wish to thank Ricardo Pellejero for his help with the literature references.
Please cite this article as: Bover J, Ureña-Torres P, Torregrosa J-V, Rodríguez-García M, Castro-Alonso C, Górriz JL, et al. Osteoporosis, densidad mineral ósea y complejo CKD-MBD (I): consideraciones diagnósticas. Nefrologia. 2018;38:476–490.


![Relationship between Chronic Kidney Disease–Mineral and Bone Disorder (CKD–MBD), renal osteodystrophy (bone changes secondary to chronic kidney disease [CKD]) and OP (associated with uraemia or age and gender of patients, among other factors). Bone strength is determined not only by bone mineral density, but also by bone quality, expressed by its determinants.94,151 Although some authors use the term “uraemic” OP,17 it is important to remember the existence of non-terminal CKD, which could be integrated within the CKD–MBD complex due to its capacity to worsen the condition. Relationship between Chronic Kidney Disease–Mineral and Bone Disorder (CKD–MBD), renal osteodystrophy (bone changes secondary to chronic kidney disease [CKD]) and OP (associated with uraemia or age and gender of patients, among other factors). Bone strength is determined not only by bone mineral density, but also by bone quality, expressed by its determinants.94,151 Although some authors use the term “uraemic” OP,17 it is important to remember the existence of non-terminal CKD, which could be integrated within the CKD–MBD complex due to its capacity to worsen the condition.](https://static.elsevier.es/multimedia/20132514/0000003800000005/v1_201811140611/S2013251418301123/v1_201811140611/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w94GCRvdQBB6xyQjMrWMzrts=)
![Estimation of fracture probability using FRAX® (fracture probability according to risk factors [CRF]). High, intermediate, and low risk levels vary according to guidelines and countries. In the United States and the United Kingdom, ≥20% 10-year probability of major osteoporotic fracture or ≥3% probability of hip fracture is considered high, 10–20% is considered an intermediate probability (densitometry [BMD, bone mineral density] and recalculation is recommended), and <10% is considered a low probability (figures based on cost-effectiveness). Some guidelines (see Fig. 4) recommend recalculating FRAX (reassess probability) using BMD for both intermediate- and high-risk patients before treating (BMD can also be used to evaluate response to treatment).26. Estimation of fracture probability using FRAX® (fracture probability according to risk factors [CRF]). High, intermediate, and low risk levels vary according to guidelines and countries. In the United States and the United Kingdom, ≥20% 10-year probability of major osteoporotic fracture or ≥3% probability of hip fracture is considered high, 10–20% is considered an intermediate probability (densitometry [BMD, bone mineral density] and recalculation is recommended), and <10% is considered a low probability (figures based on cost-effectiveness). Some guidelines (see Fig. 4) recommend recalculating FRAX (reassess probability) using BMD for both intermediate- and high-risk patients before treating (BMD can also be used to evaluate response to treatment).26.](https://static.elsevier.es/multimedia/20132514/0000003800000005/v1_201811140611/S2013251418301123/v1_201811140611/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w94GCRvdQBB6xyQjMrWMzrts=)