The Notch pathway regulates key processes in the kidney involved in embryonic development and tissue damage. Local activation of the Notch pathway has been described in many human chronic renal diseases, and has been suggested that several Notch pathway components could be considered as biomarkers of renal damage. Experimental studies done using Notch-components genetic modified mice as well as pharmacological approaches, such as γ-secretase inhibitors that block Notch pathway activation, have demonstrated the role of this pathway in renal regeneration, podocyte apoptosis, proliferation and fibroblasts activation, and induction of epithelial to mesenchymal transition of tubular epithelial cells. Recent studies suggest an interaction between Notch and NF-κB pathways in the regulation of the renal inflammatory process. Additionally, some miRNAs could regulate Notch components and down-stream responses. All these data suggest that blockade of the Notch signaling pathway could be a novel therapeutic option for renal diseases.
La vía de Notch regula procesos importantes en el riñón implicados en el desarrollo embrionario y en situaciones de agresión tisular. Así, en una gran variedad de nefropatías crónicas humanas se ha descrito una activación local de este sistema, sugiriendo que algunos de sus componentes podrían ser biomarcadores de daño renal. Los estudios realizados en modelos experimentales, modulando genéticamente componentes de la vía Notch o mediante su bloqueo farmacológico con inhibidores de la γ-secretasa, han demostrado la participación de esta vía en la regeneración renal, en la apoptosis de podocitos, en la proliferación y activación de fibroblastos y en la transición epitelio-mesenquimal de las células tubuloepiteliales. Estudios recientes sugieren una interacción entre las vías Notch y NF-κB, la cual podría jugar un papel relevante en el proceso inflamatorio renal. Por otra parte, en los últimos años se han descrito miRNA que son capaces de regular componentes de la vía Notch y modular sus respuestas. Todos estos datos indican que el bloqueo de la vía de señalización Notch podría representar una nueva opción terapéutica para la enfermedad renal.
The Notch signaling pathway is an intercellular communication mechanism used by multicellular organisms to determine the specific function and destination of cells during the formation of complex structures. The Notch route is phylogenetically conserved that regulates multiple cellular processes, such as cell proliferation, differentiation and death, control of the immune system and self-renewal of stem cells.1–3 For many years research has been carried out on the complex mechanisms involved in embryonic development, where the Notch pathway has been shown to play a key role in various organisms. This route participates in the formation of organs, such as the pancreas, and in the development of the cardiovascular system, regulating the differentiation of endothelial cells during the formation of the functional vascular tree, as well as the arteriovenous arrangement.1 In addition, it regulates the hematopoietic system, requiring the expression of canonical ligand type-Delta for the establishment of definitive hematopoiesis.2 Additionally, the Notch signaling pathway is involved in the maintenance of neuronal stem cells and in neurogenesis in the embryonic and adult brain.1 In the kidney, this pathway is activated during nephrogenesis, being inhibited in the neonatal and adult stages, and it is reactivated in situations of kidney damage.4
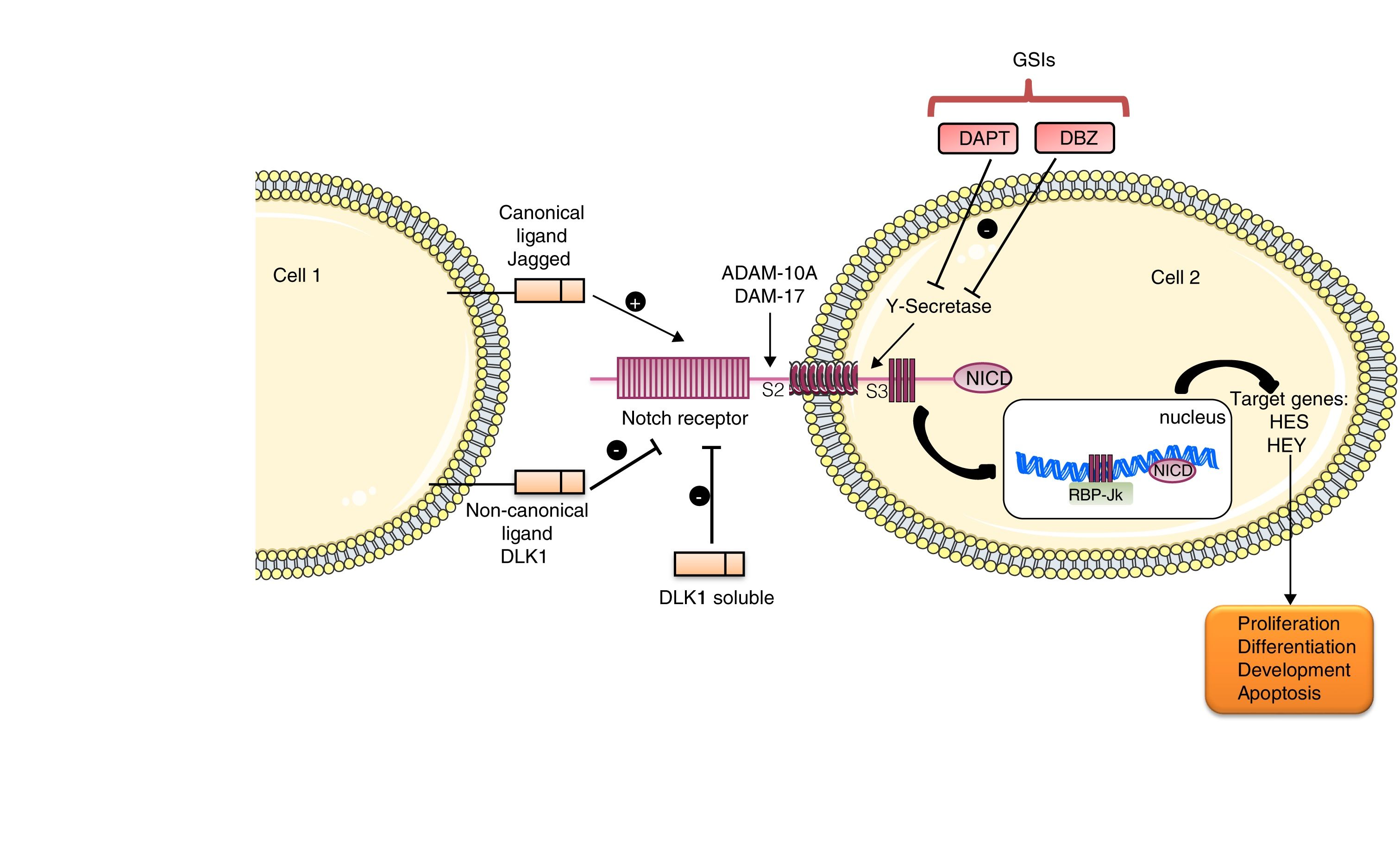
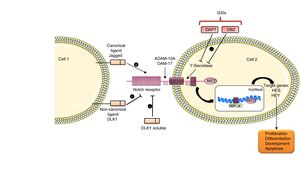
The Notch family includes a number of receptors and ligands. The Notch 1/2/3/4 receptors are transmembrane type-III proteins formed by two non-covalently associated subunits. The canonical ligands are Jagged 1/2 and Delta-type 1/2/3/45. The most important ligand is Jagged-1, which presents in its extracellular portion a DSL domain involved in receptor binding, a domain rich in cysteine that regulates the specificity of the binding and EGF-like repeats that stabilize the binding.5 This pathway is activated when the ligand binds to the Notch receptor that leads to the release of the extracellular S2 domain due to two successive proteolytic cleavages, in a process mediated by the metalloproteinases/disintegrins of the ADAM family: ADAM-10 and ADAM-17. The action of these disintegrins generates an activated form of the receptor that remains attached to the membrane, and then the γ-secretase enzyme catalyzes the second cut in the S3 domain. This final cut releases the intracellular domain of Notch (NICD), which migrates to the nucleus, where it is associated with RBP-Jκ, which in turn binds to DNA (GTGGGAA) and activates the transcription of target gene effectors of the pathway, among which they find Hes 1/2/3/4/5/7 and the repressor proteins HERP or HEY 1/2/3, which are part of the signaling in processes such as differentiation, apoptosis and proliferation 5 (Fig. 1).
In addition, there are non-canonical ligands, characterized by the lack of DSL domain of receptor binding. They are subdivided into three subgroups: the transmembrane ligands, such as DLK1 and DLK2, DNER and Jedi; those anchored to GPI, such as F3/contactin1, and those secreted, such as OSM11 and EGFL7. There are previous publications describing the controversy about the effects of ligands in the Notch pathway, since some of them activate the pathway while others inhibit it.6
Mutations in both receptors and ligands of this pathway lead to abnormalities of different severity in many tissues, including vessels, heart, kidney and hematopoietic cells. The most studied are mutations in the Notch-3 receptor or in the Jagged-1 ligand, that cause Alagille syndrome and autosomal-dominant arteriopathy, respectively.7 The mutation of the Notch-11 receptor causes bicuspid aortic valve disease, hypoplastic left heart syndrome and acute aortic syndrome. The mutations of loss of function in Notch-1 and RBP-Jκ are the cause of the Adams–Oliver syndrome, characterized by malformations of the extremities and abnormal development of the skin.1
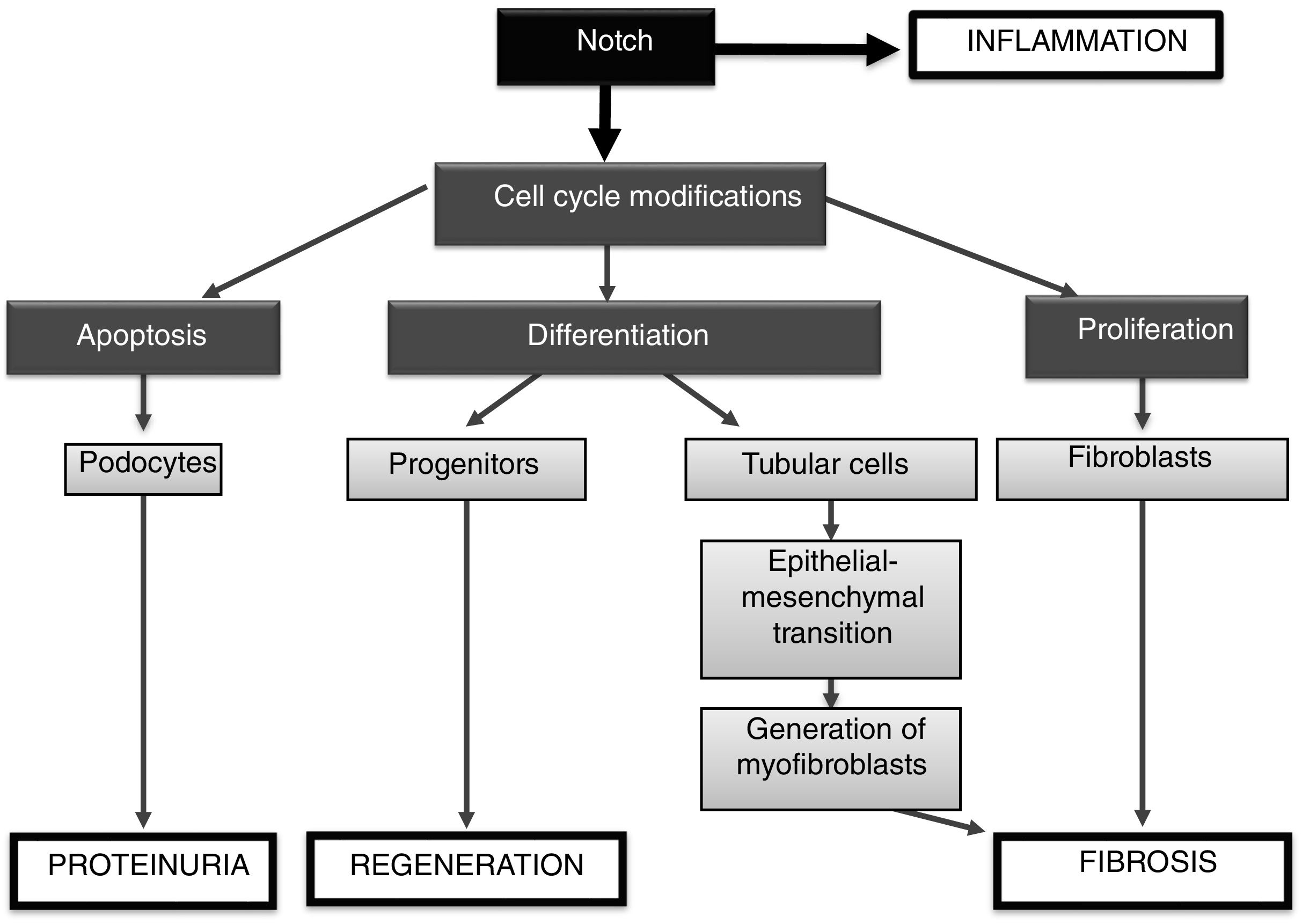
Role of the Notch pathway in kidney damageDuring embryogenesis, Jagged-1 is expressed in collecting tubules, in pre-tubular aggregates and in endothelial cells of the forming glomerulus, activating the Notch-1 and Notch-2 receptors, and in turn controlling the cellular pattern in glomerulogenesis.8 In a study conducted by the group of Susztak et al. it was described that the components of the Notch pathway are re-expressed in a wide range of renal diseases, such as glomerulonephritis of minimal changes, membranous nephropathy, lupus nephritis, hypertensive nephrosclerosis, rapidly progressive glomerulonephritis, IgA nephropathy, diabetic nephropathy and segmental focal glomerulosclerosis. They observed that the expression of Notch-1 in podocytes correlates with albuminuria and glomerulosclerosis, while the increase in NICD located in tubules is associated with tubulointerstitial fibrosis.8
These studies have suggested that some components of this pathway, such as Jagged-1 or Notch-1, could be biomarkers of kidney damage. However, our group has observed that in biopsies of patients with hypertensive nephropathy the Notch-1/Jagged-1 pathway is not activated, although we have confirmed that in progressive renal pathologies it is activated and correlates with tubulointerstitial fibrosis.9 Thus additional studies are needed in specific renal pathologies, as well as in different stages of the damage, to define whether the components of Notch could be or not defined as biomarkers of kidney damage.
Notch blockade as a therapeutic targetPreclinical studies suggest that blocking the Notch pathway by pharmacological inhibitors or soluble Notch ligands improves experimental renal damage,10–17 however the molecular mechanisms involved are not entirely clear.
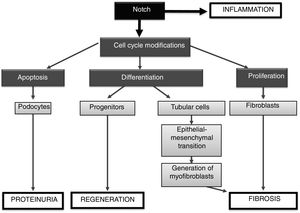
Understanding the intricate regulation of the Notch system in the damaged kidney is important, since its signaling could participate both in regeneration and in the progression of kidney damage, mainly by regulating cell proliferation. In adult kidneys there is a population of resident cells with progenitor activity that express members of the Notch family.10 In a nephrotoxic renal injury model induced by folic acid, Notch inhibition did not modify renal function in the acute phase, but renal lesions improved and fibrosis decreased in the late phase.11 Similar results have been described in other models of acute kidney injury, where Notch inhibition does not reverse the damage.12 In a model of acute tubular necrosis induced by ischemia-reperfusion, the treatment with the ligand-type Delta-4 facilitated renal recovery associated with an increase in cell proliferation.13 It should be noted that Notch activation was detected only in proliferating cells. In a model of segmental focal glomerulosclerosis induced by adriamycin, Notch inhibition reduced the loss of podocytes and improved proteinuria during the initial stages of glomerular damage, however Notch inhibition during the regenerative phases of the injury decreased the proliferation of cellular progenitors and worsened proteinuria and glomerulosclerosis.14
In cultured podocytes, Notch inhibition modulates cell death by apoptosis.15 In addition, the specific in vivo activation of Notch in the podocyte severely damages the glomerular filtration barrier,16 and, on the contrary, the specific gene deletion of the RBP-Jκ coactivator in podocytes, or the pharmacological blockade by means of the inhibition of the γ-secretase, reverts the glomerular damage and restores the glomerular filtration barrier.16 These data suggest that the specific inhibition of Notch in podocytes could be a promising therapeutic target in diseases with proteinuria that are characterized by loss of podocytes by apoptosis. The specific expression of Notch in the tubule induces epithelial differentiation, interstitial fibrosis and death of genetically modified animals.11 A recent study has shown that tubular overexpression of PGC-1α, a Notch-regulated gene, modulates renal damage by regulating fibrosis, mitochondrial dysfunction and lipid oxidation, suggesting that PGC-1α could be a key mediator of deleterious effects of Notch in the kidney.18
There is preclinical evidence suggesting that the Notch pathway regulates inflammatory processes. In experimental rheumatoid arthritis it was observed that treatment with the γ-secretase DAPT inhibitor decreased tissue damage, neutrophil infiltration, activation of NF-κB and levels of adhesion molecules such as ICAM-1 and proinflammatory cytokines.19 It has also been described that the inhibition of Notch decreased the Th17 immune response in a model of allergic asthma.20 Recently, our group has observed that treatment with DAPT decreases the presence of inflammatory cells in the kidney and reduces renal levels of proinflammatory cytokines in a model of unilateral ureteral obstruction (UUO) as well as in an experimental model of intrarenal administration of Gremlin (unpublished data). Currently these inhibitors are being evaluated in clinical trials for the treatment of several diseases, such as leukemia and Alzheimer16 (NCT00594568, NCT00762411, NCT01193868), where inflammation plays an important role. These data suggest that the beneficial effects of Notch blockade in the kidney can also be attributed to its anti-inflammatory effects. However, these inhibitors may also present certain risks. Side effects have been described in these in vivo studies, such as intestinal toxicity, which reflects the control that Notch has in the development of secretory cells versus absorbent cells within the intestinal crypts.1 Co-administration of glucocorticoids improves some of the side effects of a GSI in a mouse model of acute lymphoblastic leukemia 21 but it is not clear about the doses that could be tolerated in patients.
During the last 10 years emphasis is being placed in the use of natural antagonists as a different way of interfering with the Notch route. In addition to the canonical ligands, this pathway also has non-canonical ligands, from which the DLK1 should be highlighted. This ligand has a cleavage site by the enzyme ADAM17/TACE, which releases the extracellular portion generating a soluble form of DLK1. Both forms, soluble and membrane-anchored, have an extracellular domain with six EGF-like repeats by which it acts as a functional endogenous inhibitor.22 This antagonistic effect of Notch has been demonstrated in endothelial cells, where the over activation of DLK1 inhibits the process of angiogenesis.23 In unpublished studies, in an experimental model of kidney damage by UUO in DLK1 deficient mice we have observed an increase in the inflammatory response associated with a greater activation of the Notch-1 pathway, suggesting that DLK1 could act as an endogenous Notch antagonist regulating the renal inflammatory response.
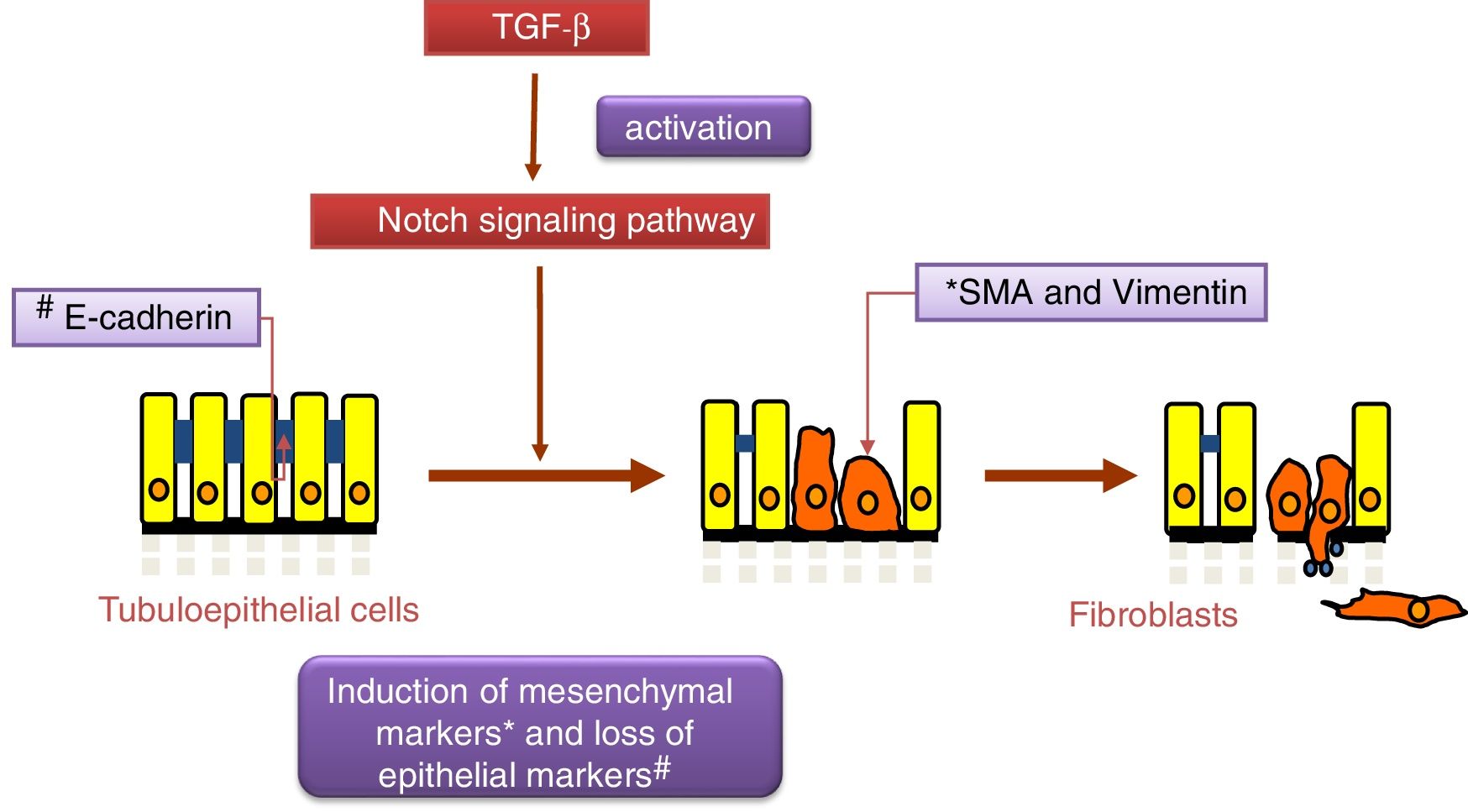
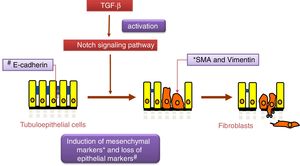
Notch and TGF-β: effects on renal fibrosis and epithelial–mesenchymal transitionThe transforming growth factor (TGF-β) is considered the principal pro-fibrogenic cytokine.24 TGF-β directly stimulates the transcription of a large number of extracellular matrix genes, inhibits the production of collagenases and induces epithelial–mesenchymal transition (EMT) (Fig. 2), a process that is involved in tubular damage and in the increase of myofibroblasts. The effects of TGF-β occur after the activation Smad proteins, its canonical pathway, or through the non-canonical pathway that uses other intracellular signaling, mainly the MAPK protein cascade.25 Gene expression of TGF-β is increased in renal pathologies and is regulated by factors that are key in the progression of renal damage, such as angiotensin II (Ang II), mechanical stress and high glucose concentrations.25 Recently, it has been described as an overlap between the signaling pathways of TGF-β and Notch 26 and both pathways participate in the control of cell differentiation during development, and in EMT during embryogenesis and cancer.27 In cultured tubuloepithelial cells the expression of Jagged-1 requires TGF-β,18 therefore the inhibition of the Notch pathway restores the changes in EMT markers induced by TGF-β.26,28 The contribution of EMT to renal fibrosis is controversial.24 At present, it is commonly accepted that the EMT process of tubular cells contributes to kidney damage, since these cells lose their polarized epithelial characteristics and their function, however their contribution to the increase in the number of myofibroblasts in the damaged kidney is controversial.24 Moreover, there are no studies demonstrating a direct effect of Notch pathway on the regulation of extracellular matrix components (ECM), such as collagens, key proteins in fibrosis. Studies in experimental models suggest that the decrease in accumulation of EMC after Notch blockade is due to its inhibitory effects on cell proliferation, particularly on activated renal fibroblasts.12
Notch and Gremlin: new anti-inflammatory therapeutic targets in renal pathologyGremlin is a gene involved in the development that is induced de novo in pathological conditions, including kidney damage.29 Gremlin belongs to the cysteine knot superfamily which antagonizes the binding of bone morphogenetic proteins (BMPs) to their receptors with a subsequent signaling blockade, the mechanism whereby processes of embryonic development and cancer is regulated.30 Several research groups, including ours, have suggested that Gremlin could be a potential therapeutic target.29 It has been suggested that Gremlin regulates fibrotic processes in different pathologies, including liver fibrosis, myocardial fibrosis and idiopathic pulmonary hypertension.29 In some pathologies, the effects of Gremlin on fibrosis have been associated with its properties as an antagonist of BMP, observing that it also regulates the Smad1/5 route.31 However, in cultured tubuloepithelial cells we have described that Gremlin activates the Smad pathway and regulates the EMT process by a mechanism independent of BMPs.32 It has been described that in endothelial cells, Gremlin binds to the receptor type 2 of vascular endothelial growth factor (VEGFR2) and regulates angiogenesis.33 We have recently described a new function of Gremlin in the kidney, it is able to activate the NF-κB pathway and regulate the acute renal inflammatory response, by a process independent of its action on BMPs, and mediated by the binding to the VEGFR2 receptor.34 Using a transgenic mouse with specific overexpression of Gremlin in tubular cells, we have observed a greater susceptibility to diabetic kidney damage after administration of streptozotocin, there was an increase in the inflammatory response with a high production of chemokines such as MCP-1.35 In patients with crescent glomerulonephritis we have observed Gremlin expression in infiltrating cells (unpublished data). To summarize, this evidence indicates that Gremlin is a proinflammatory cytokine, expanding its role as a mediator of fibrosis. Through in silico studies, it has been demonstrated that the Jagged-1 and Hes-1 genes have significant similarities with Gremlin, in terms of the structure of the promoter and of the microRNA binding elements.36 In vitro, TGF-β1 increases the expression of Gremlin, in mesangial cells, tubuloepithelial cells and fibroblasts,37 and also activates the Notch pathway.26,28,38 In addition, in renal biopsies of patients with diabetic nephropathy we have described that the induction of Gremlin expression was associated with Notch activation; this is demonstrated by immunohistochemistry in serial sections with co-localization of Gremlin, Jagged and Hes.36 In unpublished studies, we have shown that Gremlin activates the Notch pathway in the kidney and through this route it regulates the inflammatory process. These results suggest that the Notch pathway could regulate renal inflammatory processes.
The Notch pathway does not participate in renal damage mediated by angiotensin IIAng II participates actively in the progression of kidney damage, being able to regulate various pathological processes, such as inflammation and fibrosis. Drugs that block the actions of Ang II, such as angiotensin-converting enzyme (ACE) inhibitors and Ang-II receptor antagonists (ARAII), are one of the best therapeutic strategies in the treatment of progressive renal diseases, due to their actions beyond the control of blood pressure, presenting organ-protective effects.39 In in vitro studies—in various cell types, including podocytes, tubuloepithelial cells and fibroblasts—and in vivo—in several experimental models—we have described that the Notch pathway is not activated in response to Ang-II. In vitro studies clearly show that although TGF-β activates the Notch pathway in renal cells 40 Ang-II does not regulate Notch in the kidney, demonstrating a key difference between the mechanisms activated by both factors.
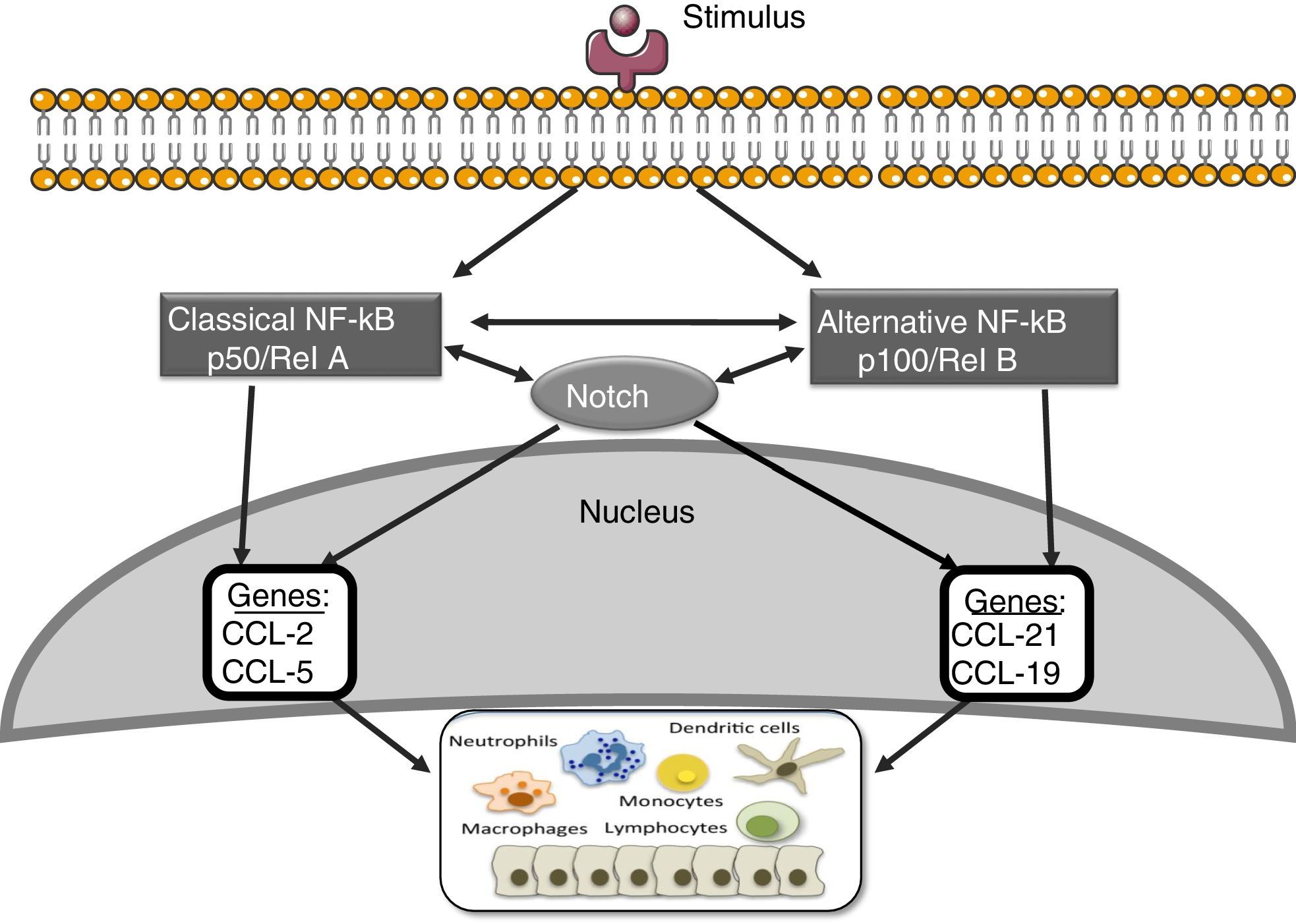
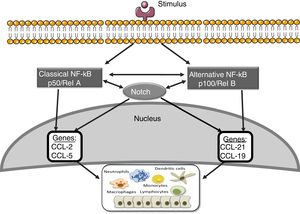
Notch and other signaling routes: NF-κBThe nuclear transcription factor kappa- B (NF-κB) plays an essential role in inflammation, immunity, apoptosis, cell proliferation and differentiation.41 There are two main routes of NF-κB activation: the classic and the alternative (Fig. 3). The activation of the classic pathway is produced by a wide variety of stimuli, such as cytokines, high glucose concentrations and advanced glycosylation products.41 The activation of the classic pathway begins with the activation of the IKK complex, formed by two catalytic subunits IKKα and IKKβ, and the regulatory subunit IKKγ/NEMO,41 which induce the phosphorylation of the inhibitory subunit IκBα, to be recognized by the ubiquitin–ligase complex, giving rise to its polyubiquitination and subsequent degradation by the proteasome 26S.41 In addition, the subunit RelA/p65 is phosphorylated and there is nuclear translocation of the RelA/p50 complex to bind to the DNA and activate the transcription of different target genes, including genes involved in the immune and inflammatory response or in tissue remodeling. The alternative NF-κB pathway is a late manifestation in response to a smaller number of stimuli. This process begins with the activation of the NIK kinase and the recruitment of IKKα to form the dimeric IKKα/IKKα complex that is phosphorylated and causes the degradation, through the proteasome, of the p100 subunit belonging to the NF-κB2 complex formed by p100/p52.41 This degradation allows the formation of the RelB/p52 complexes that are released and migrate to the nucleus to bind to the DNA, and target genes of NF-κB2 are activated, such as the chemotactic factor of lymphocyte T, CCL21.41
Several studies suggest interactions between the Notch and NF-κB pathways. The most of them have been performed on hematopoietic and tumor cells. These studies show that Notch regulates the transcription of components of the NF-κB pathway.42 In endothelial cells, Jagged-1 promotes the nuclear translocation of NICD, its physical interaction with NF-κB and the transactivation of target genes, including adhesion molecules.43 On the other hand, overexpression of p52/RelB increases Notch activation and Hes levels.44 In addition, it has been observed that the activation of the NF-κB2 promoter via the Notch pathway plays an important role in the development of rheumatoid arthritis.45 In other pathologies, such as atherosclerosis, a positive regulation has been described between the Notch and NF-κB pathways.46 We have recently observed that blockade of the Notch pathway with the DAPT inhibitor prevents the activation of NF-κB caused by Gremlin in the kidney (unpublished data). Current strategies to block NF-κB are still far from being used in the clinical setting; the use of Notch inhibitors, which also inhibits the NF-κB pathway, could be a good therapeutic option in renal inflammatory disease.
Relationship between non-coding RNA and the Notch pathwayThe non-coding RNAs (ncRNA) constitute a very heterogeneous family of transcripts that regulate gene expression, which, once they are transcribed from DNA, do not translate into proteins.47 Depending on their length they can be classified as miRNA, if they are between 18 and 24 nucleotides, and long non-coding RNA (lncRNA), if they are formed by more than 200 nucleotides. In addition, depending on their function or subcellular location, they can be classified as ribosomal RNA (rRNA), transfer RNA (tRNA), small nuclear RNA (snRNA), small nucleolar RNA (snoRNA), Piwi-associated RNA (piRNA) or small RNA of interference (siRNA). However, it is not easy to strictly separate the family of non-coding RNAs, since many of them share multiple functions.47
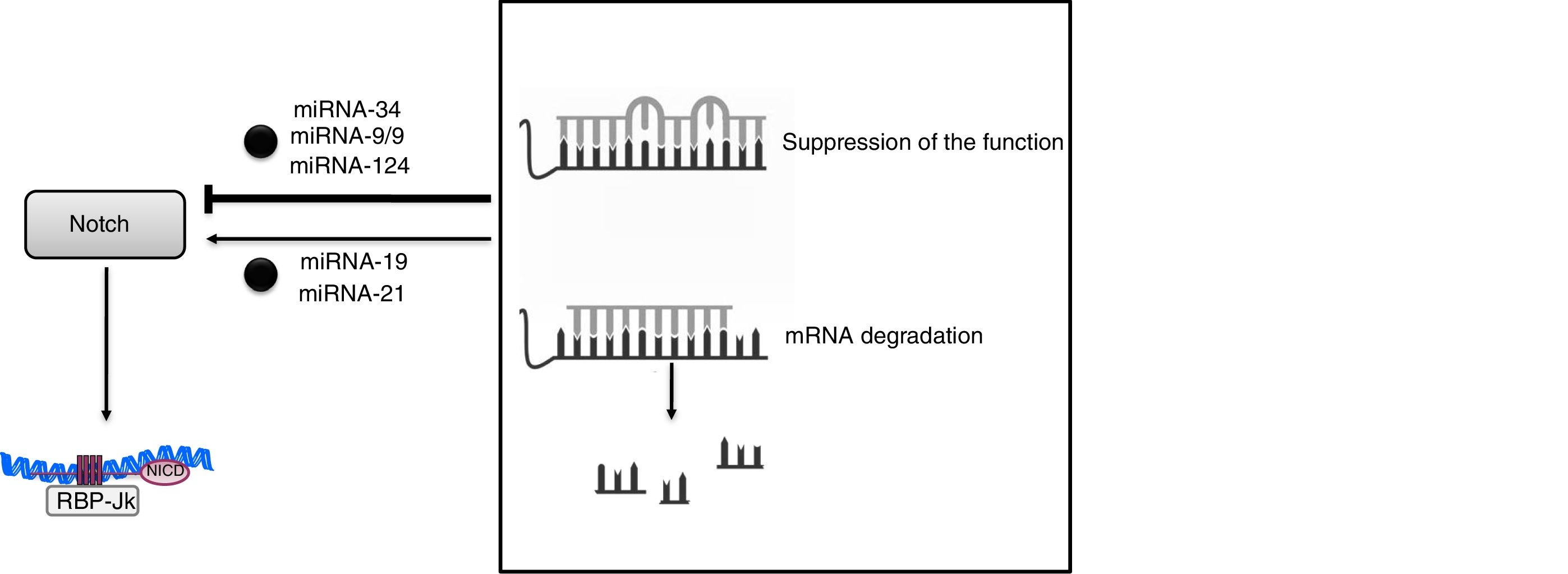
The discovery of miRNAs has brought about a revolutionary change in our understanding of post-transcriptional gene regulation. These miRNAs are very precise regulators of gene expression, and it is estimated that more than 90% of the genes in mammals could be under the control of these molecules. At present, more than 2600 miRNAs have been described in humans that are capable of regulating gene expression through the suppression of the function or degradation of messenger RNA.48 The mechanism of action is based on coupling—generally imperfect in the case of animals and perfect in that of plants—with complementary sequences, mostly present in the 3′-untranslated region (3′UTR) of its target mRNA. (Fig. 4). This unique feature means that an miRNA can regulate hundreds of mRNA and that an mRNA can be regulated by several miRNAs. These biological mediators have become specific regulators of virtually all cellular processes and act as control mechanisms of both physiological and pathological processes. Alterations in the expression and/or function of miRNAs have been associated with processes of development, aging and cell death, as well as with the pathophysiology of human diseases such as inflammation and fibrosis. Although there is redundancy in both the targets affected by the miRNA and limitations in the specificity of each of them, there is a considerable effort to use the miRNA as therapeutic tools, so that several biotechnology companies operate with this primary purpose. From the clinical point of view, the presence of certain miRNAs in blood and urine has made it possible to propose them as new early diagnostic biomarkers of diseases, including kidney diseases48 which would be a valuable improvement in this field.
Specifically in the kidney, miRNAs have been related to development, homeostasis, and renal physiological function, and they may function as negative regulators of gene expression in various stages of kidney disease.49 Thus, the miRNA-34c family interacts directly with the 3′UTR endpoint of Notch-1 and Jagged-1 (Fig. 4).50 In experimental diabetic nephropathy, overexpression of miRNA-34 inhibited Notch activation in podocytes, reducing glomerular damage and accumulation of extracellular matrix.50 The relationship between miRNAs and Notch-mediated signaling has also been described in other tissues, such as the neuronal tissue,51 where miR-9/9 * decreases Notch activity by signaling Notch-2 and Hes-1; in gastric cells, miR-124 negatively regulates Notch-1 signaling through Jagged-1.52
In addition, miRNAs can also act as positive regulators of the Notch pathway. It has been described that an increased expression of miR-19a 53 in renal cells and miR-2154 in pancreatic cancer stem cells induce Notch-mediated signaling, increasing in both cases the EMT process. These results suggest that the inhibition of both miRNAs could be beneficial for the treatment of renal and pancreatic carcinoma. In fact, miR-19a has been identified as an oncogenic miRNA in gastric and bladder cancer, and also in glioma.53 The inhibition of miR-21 suppresses the formation of tumors, metastasis and resistance to chemotherapy in cell lines.54
The main problem of gene regulation strategies based on the use of miRNAs is the specificity of the organ and the bioavailability of the products administered. If the idea is to use the miRNA as therapy for kidney diseases, it is necessary to ensure that the compound reaches its target in sufficient quantity without accumulation in other organs and systems, and it has to be eliminated effectively by the body. In addition, miRNAs are very sensitive to degradation by endonucleases present in the bloodstream, have a hydrophilic character, induce an innate immune response and could be easily eliminated by renal filtration due to their low molecular weight. Strategies based on nanotechnology have managed to overcome these obstacles and have shown encouraging results within the therapeutic field because they increase the effectiveness and/or tolerance of the organism to certain drugs, including miRNA.55 Finally, the advances made in relation to miRNA release systems have not only increased the effectiveness of these novel therapeutic agents, but have also reduced their side effects.55
In summary, the Notch signaling pathway regulates processes involved in embryonic development, homeostasis and tissue damage (Fig. 5). The activation of Notch by factors involved in kidney damage can contribute to the progression of disease, being able to: (1) activate the apoptosis of podocytes, contributing to proteinuria; (2) be involved in the renal inflammatory process, by activating signaling pathways such as NF-κB, and (3) promote renal fibrosis, by activating tubuloepithelial cells, which contribute to EMT, and eventually increase the number of myofibroblasts, or (4) to directly activate the renal fibroblasts, inducing proliferation and increasing the production of extracellular matrix proteins (Fig. 5). On the other hand, under some specific pathological situations it could contribute to renal regeneration by modulating differentiation processes (progenitors). The preclinical studies discussed here show that Notch inhibition could be a new therapeutic option for kidney disease.
Key concepts- •
The Notch pathway participates in the embryonic development of the kidney and it is reactivated in pathological situations with evident kidney damage, as has been demonstrated in a large number of human nephropathies.
- •
In preclinical studies, it has been demonstrated that blocking Notch through genetic and pharmacological approaches, improves proteinuria and renal lesions by inhibiting fibroblast proliferation and renal inflammation.
- •
Notch activation induces apoptosis of podocytes, which may contribute to proteinuria.
- •
Notch activation contributes to renal fibrosis, by inducing the proliferation of renal fibroblasts and promoting epithelium to mesenchymal phenotypic transition in tubuloepithelial cells.
- •
The interaction between the Notch pathway and NF-κB plays an important role in tissue inflammatory processes, including in renal tissue.
- •
miRNAs are capable of regulating the gene expression of components of the Notch pathway. This is the case of miRNA-34c, which regulates Notch-1/Jagged-1, or miR-19a, which regulates the EMT process.
- •
The pharmacological blockade of Notch, through the use of inhibitors of γ-secretase, provides beneficial results in human inflammatory pathologies, so it could be a therapeutic option to be evaluated in renal inflammatory diseases.
In some cases the corresponding abbreviation in English has been preserved due to its frequent use in scientific language.
| ADAM | a disintegrin and metalloproteinase |
| Ang II | angiotensin II |
| ARAII | antagonists of Ang II type I receptors |
| BMP | bone morphogenetic protein |
| DLK | epidermal growth factor-like protein Delta-like |
| DNER | Delta/Notch-like EGF-related receptor |
| DSL | domain Delta-serrate-lag-2 |
| EGF | epidermal growth factor |
| EGFL7 | secreted homologous to epidermal growth factor ligand |
| GPI | glucosilfosphatidilinositol |
| GSI | γ-secretase inhibitors |
| HERP or HEY | Hes-related repressor proteins |
| Hes | hairy/enhancer of split |
| ICAM-1 | intercellular adhesion molecule-1 |
| ACE | angiotensin converting enzyme inhibitors |
| IκB | inhibitor-kappa B |
| IKK | kinase of IκB |
| MAPK | mitogen-activated protein kinases |
| MCP | monocyte chemoattractant protein |
| ECM | extracellular matrix |
| miRNA | micro ribonucleic acid (microRNA) |
| NF-κB | nuclear factor-κB |
| NICD | active Notch intracellular domain |
| OSM11 | oncostatin M |
| PGC-1α | peroxisomal proliferation g-coactivator |
| RBP-Jκ | recombination signal-binding protein 1 for J-Kappa |
| SLN | sequence of nuclear localization e |
| TACE | TNF-α conversion enzyme (also called ADAM-17) |
| EMT | epithelial–mesenchymal transition |
| TGF-β | transforming growth factor-β |
| TNF-α | tumor necrosis factor-α |
| VEGFR2 | vascular endothelial growth factor receptor-2 |
The unpublished studies of this work were funded by the Spanish Society of Nephrology, the Health Institute CarloslanoIII and FEDER European Union Funds (ISCIII-RETIC RD16/0009, Redinren), and the projects of Chile: PAI 82140017 and FONDECYT 1160465.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Laura Marquez-Exposito, Elena Cantero-Navarro, Carolina Lavoz, Marta Fierro-Fernández, Jonay Poveda, Sandra Rayego-Mateos et al. Análisis de la vía Notch como una posible diana terapéutica en la patología renal. Nefrologia. 2018;38:466–475.