To the Editor,
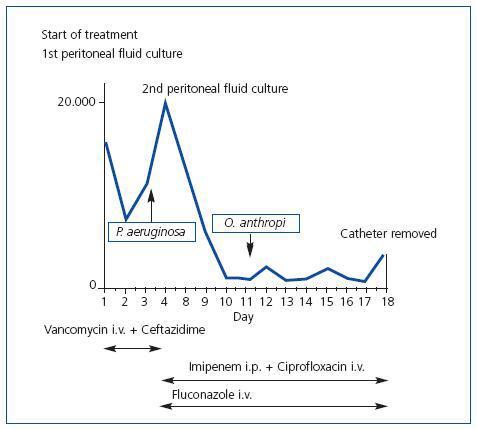
We presented the case of a 50 year old woman with terminal chronic renal failure secondary to IgA mesangial glomerulonephritis. She had a Tenckhoff type II peritoneal dialysis catheter (PD) implanted in May 2009, and started the program in September 2009. During the adaptation period she presented abdominal pain, fever of 38.2ºC and cloudy peritoneal fluid. Culture samples were taken and a cell count carried out and she started a course of antibiotics according to the our centre’s protocol: 1g intravenous vancomycin (i.v.) the first and fifth days together with intraperitoneal ceftazidime (i.p.) at a dose of 1g/day distributed during exchanges for 10 days. The physical examination revealed blood pressure (BP) of 165/90mm/Hg and painful abdomen on deep palpation with signs of peritonitis. The catheter exit site had a good appearance. Twenty-four hours after commencing empirical antibiotic treatment, the fever and abdominal pain disappeared, and the peritoneal dialysis was maintained as indicated in the established guideline (four exchanges/day). Pseudomonas aeruginosa grew in the peritoneal fluid culture and antibiotic treatment was readjusted according to antibiogram (imipenem i.p. and ciprofloxacin i.v.), with a good initial response which worsened again 48 hours later. A new culture was taken of peritoneal fluid due to the increase in cell count and reappearance of pain. This new culture grew only Ochrobactrum anthropi, with no evidence of Pseudomonas aeruginosa. The antibiotic guideline with imipenem and ciprofloxacin was maintained (for Ochrobactrum anthropi). Nonetheless, five days later she clinically worsened once more with variable behaviour of the cell count in the drained fluid. The PD catheter was therefore removed after 19 days of antibiotic treatment (Figure 1). The preventative fungicide treatment was 200mg/24 hours oral fluconazole. The patient evolved favourably and was discharged a few days later.
Peritonitis is one of the most frequent complications in PD patients and one of the causes of abandoning the technique;1 up to 10% of the cases are polymicrobial. The most frequent bacteria are gram-positive (Staphylococcus epidermidis and aureus and Streptococcus) followed by gram-negative bacteria (E. coli, Pseudomonas, Klebsiella, Enterobacter and Serratia). Peritonitis with Pseudomonas and Serratia are difficult to eradicate, they tend to be related with catheter infection and frequently the catheter has to be removed. Fungal peritonitis are less frequent but more severe and are usually associated with previous use of antibiotic therapy, immunosuppression and diabetes.
A study performed in Australia with a total of 4,675 PD patients, analysed the polymicrobial peritonitis and the most frequently isolated bacteria in the series were Staphylococcus epidermidis (21%), methicillin-sensitive Staphylococcus aureus (10%), methicillin-resistant Staphylococcus aureus (2%) and Pseudomonas aeruginosa (8%).2 This data has since been confirmed by other groups.3,4
Pseudomonas aeruginosa is an aerobic, mobile and oxidase positive gram-negative bacteria. It is considered an aggressive opportunistic pathogen, with great clinical relevance, and is often resistant to treatment. It mainly affects immunodepressed patients including catheter carriers. Biofilm or cuff catheter-associated peritonitis caused by Pseudomonas aeruginosa has been reported in patients with terminal renal failure on PD. At times, they are related with exit site infection. It is difficult to eradicate and requires the prolonged use of several antibiotics, and in up to two thirds of cases the catheter has to be removed.3
Ochrobactrum anthropi is a non-fermenting aerobic, mobile, oxidase and urease-positive, indole negative gram-negative bacillus.5,6 This micro-organism is considered an opportunistic pathogen of low virulence; it is isolated in nature and in the hospital environment it is isolated in water sources. It has also been described as an early coloniser of silicon catheters.3 Patients who use connection systems present greater probability of gram-negative bacteria infections, with a drop in the incidence of peritonitis caused by gram-positive bacteria.6 Ochrobactrum anthropi infections unrelated to PD were reported in 1980.5 They have been isolated in several types of infections: pancreatic abscesses, catheter-related bacteremias, meningitis related with contamination of cadaveric pericardial tissue, endophthalmitis, pacemaker infections and osteochondritis.7 They usually occur in immunodepressed patients (haematological, neoplastic or transplant receivers) or in permanent catheter carriers.5
Three cases of peritonitis caused by Ochrobactrum anthropi have been reported in relation with the PD. In 2000, a Spanish group published the first case in a diabetic 79-year-old woman on PD. She had suffered two previous episodes of peritonitis over the previous year. Empirical treatment of the peritonitis was commenced with vancomycin and intraperitoneal gentamicin (i.p.). Ochrobactrum anthropi was isolated in the culture, so the antibiotic was changed to ofloxacin (in accordance with the antibiogram), with a satisfactory evolution.7 The second case appeared in a 39-year-old patient 2 months after inserting the PD catheter. The initial antibiotic treatment consisted of vancomycin i.p. With no improvement on the fifth day, treatment was commenced with imipenem i.p. and i.v., as well as ceftazidime i.p., which was also associated with resolving the process.6 Another case of Ochrobactrum anthropi peritonitis has been published more recently in a 51-year-old patient, with three previous episodes of negative culture peritonitis. He presented a new symptoms of peritonitis and treatment was commenced with vancomycin and amikacin i.p. Meropenem and amikacin-sensitive Ochrobactrum anthropi grew in the culture, so the antibiotics were changed, resolving the symptoms.8
Our patient initially presented a Pseudomonas aeruginosa positive culture that responded well to antibiotic treatment, as shown in the second culture. The catheter did not have to be removed to cure the peritonitis in any of the previous cases. The most relevant aspect of this case, however, was that the catheter had to be removed with the Ochrobactrum anthropi infection. With the atypical behaviour of this peritonitis, we consider it interesting to emphasise that multiple bacteria infection can alter the immunological response of the host and, therefore, the expected virulence of each of these, as well as its response to antibiotic therapy.
Figure 1. Evolution of peritoneal fluid cell count