Antecedentes: El desgaste proteico-energético (DPE), asociado a inflamación e hiperhidratación, es común en pacientes en hemodiálisis (HD) y se asocia a mayor morbilidad y mortalidad. Objetivo: Evaluar la relación entre el estado nutricional, los marcadores inflamatorios y la composición corporal a través de bioimpedancia espectroscópica (BIS) en pacientes en HD. Métodos: En este estudio observacional, transversal, unicéntrico, realizado en un centro de HD en Forte da Casa (Portugal), participaron 75 pacientes en programa de HD. En todos los participantes se hicieron las siguientes determinaciones analíticas: hemoglobina, albúmina, proteína C reactiva (PCR) y 25-hidroxivitamina D3 [25(OH)D3]. Se calculó el índice de masa corporal (IMC) de todos los pacientes y se aplicó una versión modificada de la valoración global subjetiva (VGS) para pacientes en diálisis. El agua intracelular (AIC) y extracelular (AEC) se midió con BIS (Body Composition Monitor®, Fresenius Medical Care®) después de la sesión de HD. En el análisis estadístico se utilizó la correlación de Spearman para el análisis univariante y la regresión lineal para el análisis multivariante (SPSS 14.0). Una p < 0,05 se consideró estadísticamente significativa. Resultados: El DPE, evaluado inversamente a través de la relación AIC/peso corporal (PC), se relacionó positivamente con la edad (p < 0,001), la presencia de diabetes (p = 0,004), el IMC (p = 0,01) y la PCR (p = 0,008) y negativamente con la albúmina (p = 0,006) y la 25(OH)D3 (p = 0,007). La hiperhidratación, evaluada directamente a través de la relación AEC/PC, se relacionó positivamente con la PCR (p = 0,009) y con la VGS (p = 0,03), y negativamente con la 25(OH)D3 (p = 0,006) y el IMC (p = 0,01). En el análisis multivariante, el DPE se asoció a edad más elevada (p < 0,001), presencia de diabetes (p = 0,003), 25(OH)D3 más baja (p = 0,008), PCR más elevada (p = 0,001) y niveles de albúmina más bajos (p = 0,004). La hiperhidratación se asoció a PCR más elevada (p = 0,001) y niveles de 25(OH)D3 más bajos (p = 0,003). Conclusiones: Teniendo en cuenta estos resultados, las relaciones AIC/PC y AEC/PC, evaluadas con BIS, han demostrado ser buenos marcadores del estado nutricional e inflamatorio de pacientes en programa de HD. La BIS puede ser una herramienta útil para evaluar regularmente el estado nutricional y de hidratación en estos pacientes y puede permitir mejorar y adecuar el asesoramiento nutricional.
Background: Protein-energy wasting (PEW), associated with inflammation and overhydration, is common in haemodialysis (HD) patients and is associated with high morbidity and mortality. Objective: Assess the relationship between nutritional status, markers of inflammation and body composition through bioimpedance spectroscopy (BIS) in HD patients. Methods: This observational, cross-sectional, single centre study, carried out in an HD centre in Forte da Casa (Portugal), involved 75 patients on an HD programme. In all participating patients, the following laboratory tests were conducted: haemoglobin, albumin, C-reactive protein (CRP) and 25-hydroxyvitamin D3 [25(OH)D3]. The body mass index of all patients was calculated and a modified version of subjective global assessment (SGA) was produced for patients on dialysis. Intracellular water (ICW) and extracellular water (ECW) were measured by BIS (Body Composition Monitor®, Fresenius Medical Care®) after the HD session. In statistical analysis, Spearman’s correlation was used for the univariate analysis and linear regression for the multivariate analysis (SPSS 14.0). A P value of <.05 was considered statistically significant. Results: PEW, inversely assessed through the ICW/body weight (BW) ratio, was positively related to age (p<.001), presence of diabetes (p=.004), BMI (p=.01) and CRP (P=.008) and negatively related to albumin (p=.006) and 25(OH)D3 (p=.007). Overhydration, assessed directly through the ECW/BW ratio, was positively related with CRP (p=.009) and SGA (p=.03), and negatively with 25(OH)D3 (p=.006) and BMI (p=.01). In multivariate analysis, PEW was associated with older age (p<.001), the presence of diabetes (p=.003), lower 25(OH)D3 (p=.008), higher CRP (p=.001) and lower albumin levels (p=.004). Overhydration was associated with higher CRP (p=.001) and lower levels of 25(OH)D3 (p=.003). Conclusions: Taking these results into account, the ICW/BW and ECW/BW ratios, assessed with BIS, have proven to be good markers of the nutritional and inflammatory status of HD patients. BIS may be a useful tool for regularly assessing the nutritional and hydration status in these patients and may allow nutritional advice to be improved and adjusted.
INTRODUCTION
Protein-energy wasting (PEW), associated with inflammation and overhydration, is common in patients on haemodialysis (HD) and is associated with high morbidity and mortality.1. C-reactive protein (CRP) is an independent predictor of cardiovascular risk and mortality in advanced chronic kidney disease (ACKD).1
Subjective global assessment (SGA) is the tool most commonly used for assessing nutritional status and is valid in HD patients.3 Anthropometric data, such as the body mass index (BMI), are indicators of nutritional status in these patients,2 but they have their limitations when considered alone. Serum albumin has been widely used as a nutritional marker in HD patients and is one of the most sensitive mortality markers4 and a major marker of inflammation.1,5 For this reason, a decrease in albumin may reflect an inflammatory state instead of PEW1 and as albumin decreases with fluid overload, it is less relevant in HD patients.1 The anti-inflammatory effects of vitamin D are well documented6 and several authors, including our research group, have reported a strong correlation between the deficiency of 25-hydroxyvitamin D3 [25(OH)D3] and mortality in both the general population,7,8 and in HD patients.9,10
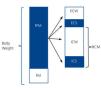
Bioimpedance spectroscopy (BIS) seems to be a valid method for assessing and monitoring hydration and nutritional status in HD patients11 and, apart from determining the individual fluid status (total body water, intra and extracellular water [ICW/ECW]) independently,12it analyses body composition (body cell mass, lean mass and fat mass) simply, objectively and non-invasively.13 Total body water can be divided into ICW and ECW and fat-free mass (FFM) in ECW and body cell mass (BCM), which includes ICW (Figure 1). The ECW compartment predominantly shows overhydration,13,14 which often exists in HD patients and is associated with inflammation and an increased risk of mortality.15 BCM is the compartment that shows nutritional status16 and, as it is not greatly affected by changes in hydration, which mainly occur in the compartment that shows overhydration (ECW), it gives us more information than FFM in these patients. The ICW compartment comprises 72% of BCM and is not affected by iso-osmotic changes that occur in the ECW compartment. Furthermore, ICW is often used to estimate BCM, which reflects nutritional status.13,14 To assess nutritional status and hydration, two relationships can be used: ICW/body weight (BW), which shows nutritional status (the higher it is, the better nourished the patient is and vice-versa), and ECW/BW, which reflects overhydration.14,17
The main aim of this study was to assess the relationship between nutritional status, inflammation markers and body composition, assessed through BIS, in maintenance HD patients.
METHODS
Study design
An observational, cross-sectional, single centre study of a cohort of maintenance HD patients.
Patients
We included 75 patients on HD three days a week (with an online haemofiltration technique), who had been stable for at least three months, 51% male, 29% diabetic, and all older than 18 years old. The aetiologies of chronic renal failure were: diabetic nephropathy (n=17), hypertensive nephrosclerosis (n=13), chronic glomerulosclerosis (n=9), polycystic kidney disease (n=4), chronic pyelonephritis (n=6), other (n=13), unknown (n=13). The other clinical and nutritional characteristics of the population studied are displayed in Table 1.
All patients were dialysed with high-flux (Helixone®, Fresenius®) membranes and ultrapure water in accordance with the criteria of ISO regulation 13959:2009 - Water for haemodialysis and related therapies. The mean time on HD was 32.3±27.1 months. One of the limitations recognised by the manufacturer of the Body Composition Monitor consists of its use in patients with major amputations of limbs or with pacemakers, since it does not guarantee that the measurements will be accurate in these patients. Therefore, these patients were excluded from the study.
Laboratory tests
The following laboratory tests were carried out: haemoglobin, albumin, CRP and 25(OH)D3. These were calculated before the midweek dialysis session, close to the day on which the BIS was carried out.
Albumin was measured using the colourimetric technique with bromocresol purple (reference value >4.0g/dl) and CRP was obtained by the immunoturbidimetric method. Serum 25(OH)D3 was measured by radioimmunoassay (IDS, Boldon, United Kingdom). The reference value for 25(OH)D3 was 10-60ng/ml.
Analysis of body composition
In all patients BIS was carried out using the Body Composition Monitor (Fresenius Medical Care Deutschland GmbH, Germany), which takes measurements at 50 frequencies in a range of 5 to 1000KHz. The measurement was performed approximately 30 minutes after the midweek HD session, with four conventional electrodes being placed in the patient, who was lying in the supine position: two in the hand and two in the foot contralateral to the vascular access. Regarding the quality of measurements, all exceeded 95%. The manufacturer of the Body Composition Monitor (Fresenius Medical Care) indicated that 30 minutes after the HD session, the balance between intra-and extracellular fluid was restored and no significant differences in relation to pre-dialysis values18 were observed. Parameters obtained directly through BIS that were used in this study were ICW and ECW. PEW, represented by the ICW/BW ratio, and overhydration, represented by the ECW/BW ratio were analysed.
Subjective global assessment
We used the modified version of the SGA for dialysis patients.19 The data were obtained taking into account changes in BW, eating habits, the presence of gastrointestinal symptoms, functional activity and the presence of comorbidities. We performed a brief physical test to assess the loss of muscle mass and fat mass and the presence of oedema. Each component of the SGA was assigned a score of 1 (normal) to 5 (very severe). A final rating was attributed to each patient after adding up all the scores of the different components of the SGA; the higher the score, the greater the risk of PEW.
Anthropometric parameters
The following data were collected: height (m) measured with a precision telescopic stadiometer (Seca 222®), dry weight (kg) measured with a calibrated scale (Soehnle® S20) and BMI (kg/m2).
Statistical analysis
Categorical variables are expressed as frequencies, normally distributed variables are expressed as mean ± standard deviation and variables whose distribution is not normal are expressed as a median (interquartile range). We used Spearman’s correlation for univariate analysis and performed a multivariate linear regression analysis (confidence interval 95%).
For statistical calculations, we used the SPSS 14.0 software (SPSS Inc., Chicago, IL, USA). A p value of <.05 was considered to be statistically significant.
RESULTS
In our study, BMI was not significantly related to nutritional or inflammatory parameters.
Overall, 16 patients (22%) had albumin below 4.0g/dl. The mean value of serum albumin was above 4.0g/dl and the median CRP was below 1mg/dl, in accordance with the K/DOQI guidelines.3 Serum 25(OH)D3 was below the desirable values (<30ng/ml) in 59 (80%) patients.
In univariate regression analysis, PEW, assessed inversely by the ICW/BW ratio, was positively associated with age, diabetes, BMI, CRP and ferritin. PEW was negatively related to albumin and 25(OH)D3 (Table 2). Moreover, a negative correlation was found between serum albumin and CRP (r=-.49, p<.001) and lower concentrations of 25(OH)D3 were associated with higher levels of CRP (r=-.29, p=.03).
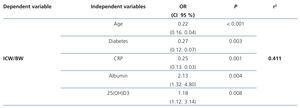
The multivariate analysis shows that PEW is associated with older age, presence of diabetes, lower levels of 25(OH)D3 and albumin and higher CRP levels (Table 3).
In the univariate analysis, overhydration, assessed by the ECW/BW ratio, was positively related to CRP and SGA, and negatively to serum 25(OH)D3 and BMI (Table 2). In the multivariate analysis, overhydration was associated with higher levels of CRP and lower 25(OH)D3 (Table 4).
DISCUSSION
In this study, a worse nutritional status (lower ICW/BW) was associated with older age, diabetes and inflammatory markers. Diabetes mellitus has been identified as a risk factor for PEW in HD patients.20 The value of albumin as a marker of nutritional status has been questioned because its concentration is affected by non-nutritional parameters such as hydration status, loss of albumin in the urine and acidosis,21 infection or inflammation, with the latter factor being the most important cause leading to the decrease in albumin.1,22 through BIS analysis, we demonstrated that a worse nutritional status was associated with lower levels of albumin and increased inflammation (higher levels of CRP and ferritin). Like other authors,23 our results also indicated a negative correlation between serum albumin and CRP, which suggests that lower albumin levels that are observed in patients with PEW may be secondary to inflammatory processes. According to the data obtained, lower levels of 25(OH)D3 were associated with poorer nutritional status and its deficiency contributes to the development of chronic inflammation because of its association with higher CRP concentrations.24 In our study, lower levels of 25(OH)D3 were associated with higher CRP levels. In this regard, it has been shown that this vitamin supplement leads to reduced CRP levels.25
In accordance with these results, the ICW/BW ratio analysed through BIS appears to be adequate for monitoring the nutritional status of maintenance HD patients, since a worse nutritional status is significantly associated with common conditions in HD patients such as: advanced age, diabetes, inflammation and deficiency of 25(OH)D3.
Our data on BMI are in line with recent studies,26,27 which have shown a progressive increase in BMI in patients with ACKD, as well as a greater number of overweight and obese patients.28 In this study, we found a negative association between ICW/BW and BMI, which means that better nourished patients were those with lower BMI values. It should be noted that for patients in this study, in accordance with BMI, only 4% were underweight. In maintenance HD patients, there is an unusual relationship (called a “reverse epidemiology”) between BMI and survival, with increased mortality in patients with low BMI (BMI <20kg/m2 [29]) and increased survival in overweight patients (BMI ≥27.5kg/m2 [30], BMI >25kg/m2 [29,31]) and obese patients (BMI >30kg/m2 [31]).29,30,32 It should be noted that BMI does not discriminate between lean mass, fat and water, it only determines a ratio between weight and height and not all patients who have a high BMI have it at the expense of fat.33 However, the use of BMI alone has limitations in assessing the nutritional status of these patients34 because it can hide PEW in overhydrated patients.2
With regard to the SGA, most patients had risk of malnutrition or mild malnutrition, very few had moderate and none severe. It is important to highlight that in the modified version of SGA for dialysis patients, all patients who had been on HD for more than a year, even with normal clinical history parameters and physical examination, obtained a score of 9, which classified them as having nutritional risk or being at risk of mild malnutrition. This situation explains the high prevalence of malnutrition (assessed by SGA) observed in our population, as the mean time on dialysis was greater than 2 years (68% of patients were on HD for more than a year). Similar results have been reported by other authors.35 In our study, the SGA was not related to the ICW/BW ratio, which may have been because there were only two patients with moderate and none with severe malnutrition. Although this nutritional score tool is useful for differentiating severely malnourished patients from those with an adequate nutritional status, it may not be a reliable indicator of the degree of malnutrition.1,19,36
Nonadherence to fluid restrictions can lead to fluid overload37 and this is one of the most difficult aspects of the HD treatment regimen,15 with a prevalence of perceived failure by patients ranging from 30% to 74%.38,39 In our study, overhydration was associated with higher CRP concentrations. This association has previously been described in dialysis patients.40,41 The link between inflammation and expansion of extracellular volume is probably caused by intestinal oedema that facilitates the translocation of bacterial toxins.42,43Furthermore, our results show that patients with overhydration had poorer nutritional status (higher SGA and lower 25(OH)D3). It has been reported that the prevalence of PEW, evaluated through the SGA, decreased in patients in whom hydration improved (ECW decreased and ICW increased), and vice-versa.44 The same association was found using a different nutritional score (the Bilbrey nutritional index).45 Deficiency in 25(OH)D3 has been associated with inhibition of the renin-angiotensin46 pathway, which may be related to overhydration. The negative correlation observed between overhydration and BMI suggests that higher BMI values were associated with greater accumulation of body fat. This supports the fact that patients with better nutritional status were those with normal weight, according to the BMI classification.Therefore, hydration status, assesd through regular BIS analysis, can allow detecting changes in both nutritional and inflammatory status. As has already been described by other authors,11 BIS is an important technique to assess nutritional and hydration status and has proved to be as accurate as the reference methods considered as the gold standard.47 Some authors believe that the spectroscopic measurement technique has high reproducibility and specificity.47,48 In patients on HD, it has been used to assess dry weight,49,50 but it is also valid for assessing body composition, nutritional status,14,51 and overhydration.52,53 Moreover, it has been shown that BIS, through measuring hydration status, may be suitable for assessing the prognosis of HD patients.54,55
The limitations of our study are its sample size and its cross-sectional, single centre design. However, successive measurements with BIS over time may be very useful for assessing the trend of nutritional status, inflammation and hydration in HD patients, which would allow therapy to be optimised and nutritional advice to be adjusted.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Patients characteristics
Table 2. Spearmans correlation coefficients between intracellular water/body weight and extracellular water/body weight with nutritional and inflammatory markers (univariate analysis)
Table 3. Association between intracellular water/body weight and different parameters, including markers of inflammation and nutrition (multivariate analysis)
Table 4. Associations between extracellular water/body weight and markers of inflammation and nutrition (multivariate analysis)
Figure 1. Body composition