Antecedentes: Existen pocos datos acerca de la prevalencia de los trastornos del metabolismo óseo y mineral (MOM) y su forma de manejo en pacientes con enfermedad renal crónica en Argentina. Objetivos y métodos: Mediante una encuesta realizada en 2010 en centros de diálisis, determinamos la prevalencia y las formas de tratamiento de los trastornos del MOM en Argentina y los comparamos con los datos publicados por otros grandes estudios poblacionales. Consignamos las características de los centros de diálisis y de los pacientes participantes, la frecuencia de las determinaciones y los resultados individuales de los marcadores bioquímicos del MOM y el tipo de manejo utilizado para controlar la hiperfosfatemia y el hiperparatiroidismo secundario. Resultados: Participaron 1210 pacientes de 25 centros de diálisis de 10 provincias de Argentina (lo cual representaba el 4,7 % de la población prevalente en diálisis del país en 2010). La población estudiada tenía una edad media de 55,3 ± 17,6 años, 60,8 % eran varones, el 3,3 % en diálisis peritoneal y el 29,1 % eran diabéticos. El 100 % de los centros determinaban calcemia y fosfatemia mensualmente, el 60 % hormona paratiroidea intacta (PTHi) semestralmente, el 36 % cada 3 o 4 meses y el 4 % de forma anual. Según las recomendaciones de K/DOQI, el 51,6 % de los pacientes tenían niveles adecuados de calcio (8,4-9,5 mg/dl), el 51,6 % de fósforo (3,5-5,5 mg/dl) y el 21,1 % de PTHi (150 a 300 pg/ml). El 24,4 % tenían PTHi < 150 pg/ml y el 54,5 % > 300 pg/ml, con un 28,3 % con valores de PTHi > 600 pg/ml y un 13,3 % > 1000 pg/ml. Estos datos diferían de los publicados por el estudio DOPPS II, donde el 51,1 % de los pacientes presentaban PTHi < 150 pg/ml, y solo un 26,7 % PTHi > 300 pg/ml. El 83,6 % utilizaban un captor del fosfato basado en calcio, el 5,6 % sevelamer y el 4,0 % compuestos con aluminio. Para el control del hiperparatiroidismo se utilizaba predominantemente calcitriol oral o endovenoso (50,5 %), con un pequeño porcentaje de pacientes recibiendo paricalcitol o doxercalciferol. Conclusiones: El presente estudio muestra una elevada prevalencia de hiperparatiroidismo secundario, lo cual difiere de lo publicado por otros grandes estudios poblacionales. Existe una elevada proporción de pacientes con marcadores del MOM por fuera de los niveles sugeridos por K/DOQI. Para el control de la hiperfosfatemia y el hiperparatiroidismo, se continúan utilizando mayormente captores del fosfato basados en calcio y calcitriol, respectivamente.
Background: There are few data in Argentina on the prevalence and management of bone and mineral metabolism (BMM) in patients with chronic kidney disease (CKD). Objectives and methods: A survey was carried out in dialysis units in 2010 to measure the prevalence of and types of treatments for BMM disorders in Argentina. The data obtained was then compared to the published results from other large population studies. We recorded characteristics of dialysis centres and participating patients, the frequency of measurements and individual results for BMM biochemical markers, as well as the type of management used to control hyperphosphataemia and secondary hyperparathyroidism. Results: 1210 patients from 25 dialysis centres in Argentina participated in the study (representing 4.7% of the country’s prevalent dialysis population in 2010). The mean patient age was 55.3±17.6 years, 60.8% were male, 3.3% were on peritoneal dialysis and 29.1% suffered diabetes. In all centres, phosphataemia and calcaemia were measured on a monthly basis, 60% of centres measured intact parathyroid hormone (iPTH) every 6 months, 36% every 3 to 4 months, and 4% annually. As recommended by K/DOQI, 51.6% of patients had adequate levels of calcium (8.4-9.5mg/dl), 51.6% had adequate phosphorus (3.5-5.5mg/dl) and 21.1% displayed acceptable iPTH levels (150-300pg/ml). 24% had iPTH <150pg/ml and 54.5% >300pg/ml. iPTH >600pg/ml was present in 28.3%, and 13.3% had values >1000pg/ml. These figures differed from those published by the DOPPS II study, in which 51.1% of patients had iPTH <150pg/ml, and only 26.7% had iPTH >300pg/ml. Calcium-based phosphate binders were used in 83.6% of the patients, 5.6% used sevelamer and 4.0% used aluminium-containing compounds. To achieve control of hyperparathyroidism, oral or intravenous calcitriol was predominantly used (50.5%) with a small percentage of patients receiving paricalcitol or doxercalciferol. Conclusions: The present study shows a high prevalence of secondary hyperparathyroidism, which differs from that published by other large population studies. There was a high proportion of patients with BMM markers outside the ranges suggested by K/DOQI. Mainly phosphate binders based on calcium and calcitriol continue to be used for the management of hyperphosphatemia and hyperparathyroidism respectively.
Secondary hyperparathyroidism, far from being an epiphenomenon that accompanies chronic kidney disease (CKD), is a disorder that directly affects the quality of life of people with kidney failure, while some of its components, such as hyperphosphatemia or cardiovascular calcifications are related to mortality.1-3
The spectrum of bone and mineral metabolism (BMM) disorders has varied over time in relation to different factors such as the causes of CKD, the age of the dialysis population, kidney transplantations and primarily, the different options that have been introduced and have become available for treating these diseases.4-7
Over the past four decades, we have witnessed the evolution from predominantly low remodelling forms of BMM disorders, related to aluminium overload (in the 1970’s and 1980’s), to mixed and high remodelling forms due to secondary hyperparathyroidism (in the 1990’s), and finally to the last decade (the year 2000 to present), which has been dominated by low remodelling forms with adynamic bone disease probably related to an increase in the prevalence of diabetes as a cause of CKD and the use of different forms of treatment for hyperparathyroidism.4,5,8-10
In the latter period, data from DOPPS I and II (Dialysis Outcomes and Practice Patterns Study) show the evolution of mineral metabolism disorders in dialysis patients in different regions of the world. Of these, 51.1% had values below 150pg/ml, while only 26.7% had intact parathyroid hormone (iPTH) >300 pg/ml.11,12
Unfortunately, there is limited data on the prevalence of these disorders in patients in Latin America and practically no information is available in the literature on managing BMM disorders in patients with CKD in this region. In a cooperative study using data from 1209 bone biopsies taken in the 1990’s from CKD patients on dialysis in several Latin American countries, Jorgetti et al. demonstrated that the prevalent histological forms in Brazil, Argentina and Uruguay were the low remodelling forms, such as osteomalacia and mixed forms related to high aluminium deposits, while in Portugal and Spain, high remodelling forms related to secondary hyperparathyroidism were prevalent.13 More recently, we reported the prevalence of fibrous osteitis related to secondary hyperparathyroidism in bone biopsies taken from patients from central Argentina and there was an increase in cases of osteitis fibrosa at the end of follow-up.7
Beyond the factors known to be responsible for this disease, there are geographical differences in its spectrum attributed to factors specific to patients and access to different treatment modalities. It is important to know the prevalence of various predominant forms in the region in order to adopt the most appropriate health policies. Through a survey carried out in various dialysis centres in Argentina, we analysed the prevalence of BMM disorders in patients with CKD and the different treatment modalities used in the setting.
METHODS
28 dialysis centres from different provinces of Argentina were invited to participate in a survey. This was aimed at finding out the individual data of the dialysis population and treatment centres.
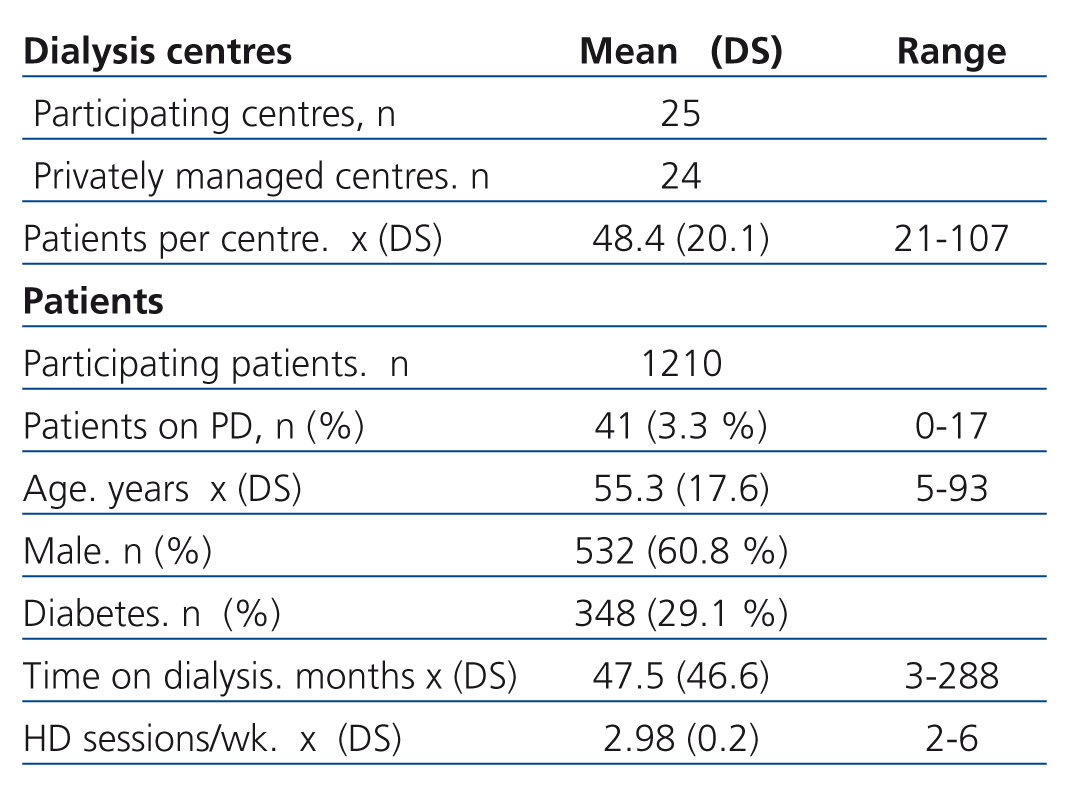
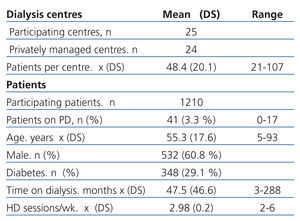
The selection of participating centres was carried out using the list provided by the Sistema Nacional de Información de Procuración y Trasplante de la República Argentina (SINTRA), which includes all the units in Argentina (http://sintra.incucai.gov.ar/). The survey was designed to include a broad spectrum of the dialysis population in Argentina in both urban and rural areas, respecting the proportion of private (96%) to public centres in the country. Of the 28 dialysis centres invited to participate, 25 (89.3%) from 10 of the country’s 23 provinces responded by sending their data. One centre declined to participate without giving a reason and the other two did not send their data within the requested time (Table 1).
Fourteen centres were from the province of Córdoba, two were from Catamarca and Santa Fe and there was one from Buenos Aires, Salta, Río Negro, San Luis, Entre Rios, Corrientes and La Rioja. Therefore, most of the centres were from central and north-western Argentina.
Data was received from 1210 dialysis patients. In 2010, the prevalence of dialysis patients in Argentina was 25 979, and as such, the data provided were from approximately 4.7% of the total patients in the country.14
Patients of both sexes were included and there was no age limit. 17.6% (n = 213) were incident patients, while 82.4% (n = 997) were prevalent patients. We excluded patients who had been on dialysis for less than three months or whose data was incorrectly recorded.
Table 1 shows the characteristics of the patients reported by the 25 dialysis centres. The average number of patients per centre was 48.4±20.1. 3.3% were treated by peritoneal dialysis. Only seven of the 25 centres had peritoneal dialysis patients. 60.8% of patients were male and the mean age of the population was 55.3±17.6 years at the time of the study, while the mean time on dialysis was 47.5±46.6 months. 29.1% of patients had diabetes as the primary cause of CKD. 95.8% of patients were on haemodialysis with a tri-weekly regimen, 2.9% underwent haemodialysis twice a week, 10 patients four times a week and one patient had six weekly sessions (Table 1).
Individual data were recorded without patient identification and were communicated anonymously. The data analyst was unaware of which centre they belonged to.
The questions asked were:
- Characteristics of the dialysis population and the participating centres. The following data were recorded: age, sex, cause of CKD (diabetes YES or NO), time on dialysis measured in months, type of dialysis received (haemodialysis or peritoneal dialysis) and the number of weekly sessions of haemodialysis.
- Frequency of mineral metabolism biochemical marker measurements (serum calcium and phosphorus, alkaline phosphatase, parathyroid hormone and 25 (OH) cholecalciferol D3 [25 (OH) D3, calcidiol]: monthly, quarterly, four-monthly, six-monthly or annually.
- We requested a report of the individual results of the mineral metabolism biochemical analyses for July 2010 or the last measurement recorded for each patient of the dialysis centre. We requested non-albumin corrected serum calcium results in mg/dl, serum phosphorus in mg/dl, alkaline phosphatase in IU/l, iPTH intact molecule in pg/ml and 25 (OH) D3 in ug/ml.
- In addition, we requested data on the type of treatment used and the daily dose of medication to control hyperphosphataemia (phosphate binder) and secondary hyperparathyroidism (calcitrol with route of administration, vitamin D analogues or invasive methods, such as surgical parathyroidectomy or injection of alcohol into the parathyroid gland).
As a reference on the prevalence of BMM disorders in the dialysis population in other countries, we took data from DOPPS, considered to be one of the most extensive and disseminated studies on the subject.11,12 As in most published studies, DOPPS uses, as a reference, values for BMM markers in patients with stage 5D chronic renal failure, provided by KDOQI guidelines.15,16 In order to compare our results with those of DOPPS, we used KDOQI values in our study.
The survey was sent and responded to electronically. Data not reported by the centres on survey forms were considered unknown.
The study was supervised and approved by the Comité de Revisión de Trabajos de Investigación y de Ética del Hospital Privado-Centro Médico de Córdoba, Argentina, and COEIS (Consejo de Evaluación Ética de la Investigación en Salud, http://www.cba.gov.ar/coeis).
Statistical analysis
Data were analysed through the study of basic statistics. To compare two normally distributed continuous variables in two different groups of patients, we used the Student’s t test for unpaired data. We compared two nominal variables using the Χ2 test. The computer-based statistical program used was Stat View version 4.5.
RESULTS
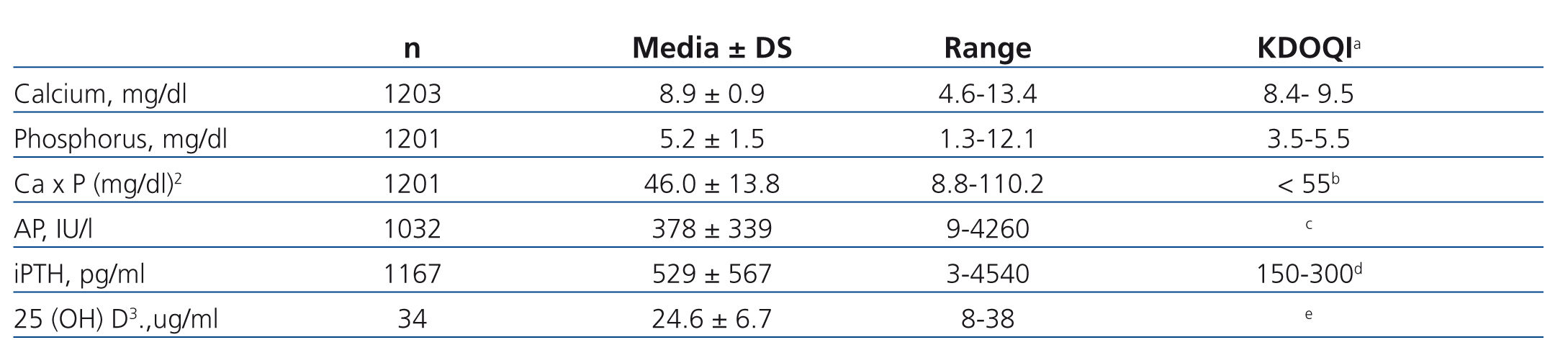
Each month, all centres measured serum phosphorus and calcium, 36% of centres measured alkaline phosphatase levels monthly and 25% quarterly, while 20% did so every six months. Most centres (60%) measured iPTH every six months, while 36% did so quarterly or three-monthly, and one centre measured PTH once a year. Only one centre reported regular measurement of 25 (OH) D3.
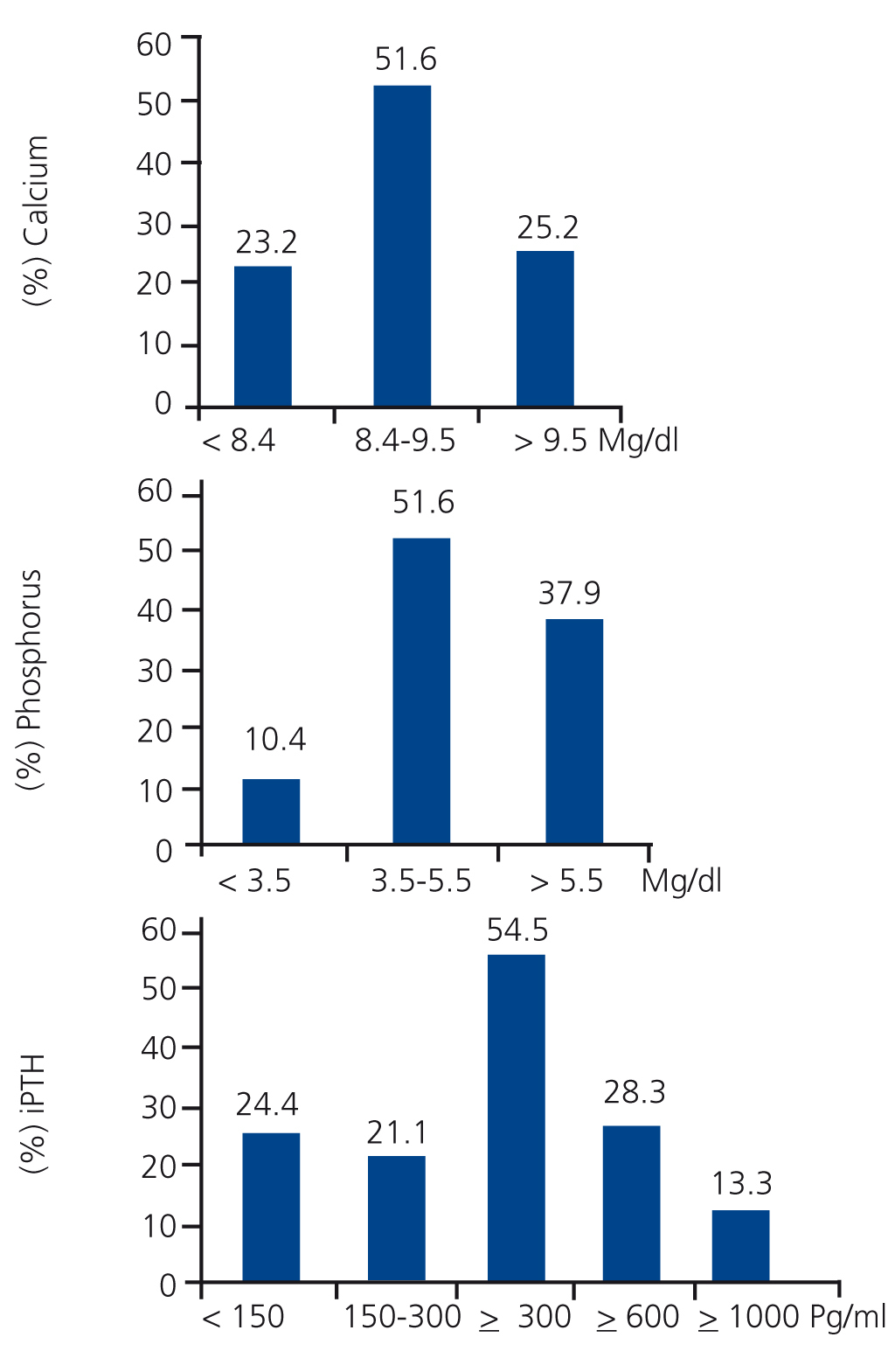
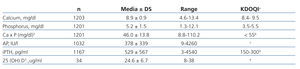
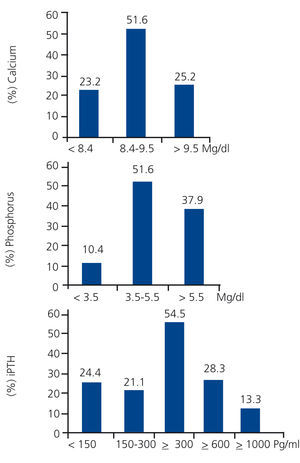
The mean level of serum calcium was 8.9±0.9mg/dl, with a range between 4.6 and 13.4mg/dl. 51.6% (n = 621) of patients had adequate levels of calcium according to KDOQI guidelines (8.4 to 9.5mg/dl), while 25.2% (n = 303) were above and 23.2% (n = 279) were below the values suggested by the guidelines for mineral metabolism (Figure 1).14 Mean phosphataemia was 5.2±1.5mg/dl, with a minimum of 1.3mg/dl and a maximum of 12.1mg/dl. In 51.6% (n = 620) of patients, phosphataemia was adequate according to KDOQI guidelines (3.5-5.5mg/dl), in 10.4% (n = 125) it was below and in 37.9% (n = 456) above the suggested levels (Figure 1). Mean alkaline phosphatase was 378±339, with a minimum of 9 and a maximum of 4260IU/l. The mean level of iPTH was 529±567, with a range from 3 to 4540pg/ml. Only 21.1% of patients (n = 246) had levels suggested by the KDOQI guidelines (150-300pg/ml), 24.4% (n = 284) had values below those suggested by the guidelines and 54.5% (n = 636) above 300pg/ml (Figure 1). Importantly, 28.3% of patients had iPTH values ≥600pg/ml and 13.3% had values ≥1000pg/ml. According to the values suggested by the KDIGO guidelines, 47.3% (n = 552) had adequate levels (150-600) and 28.3% (n = 330) had values that were consistent with hyperparathyroidism (Figure 1).15 Only 34 measurements of 25 (OH) D3 were reported (Table 2).
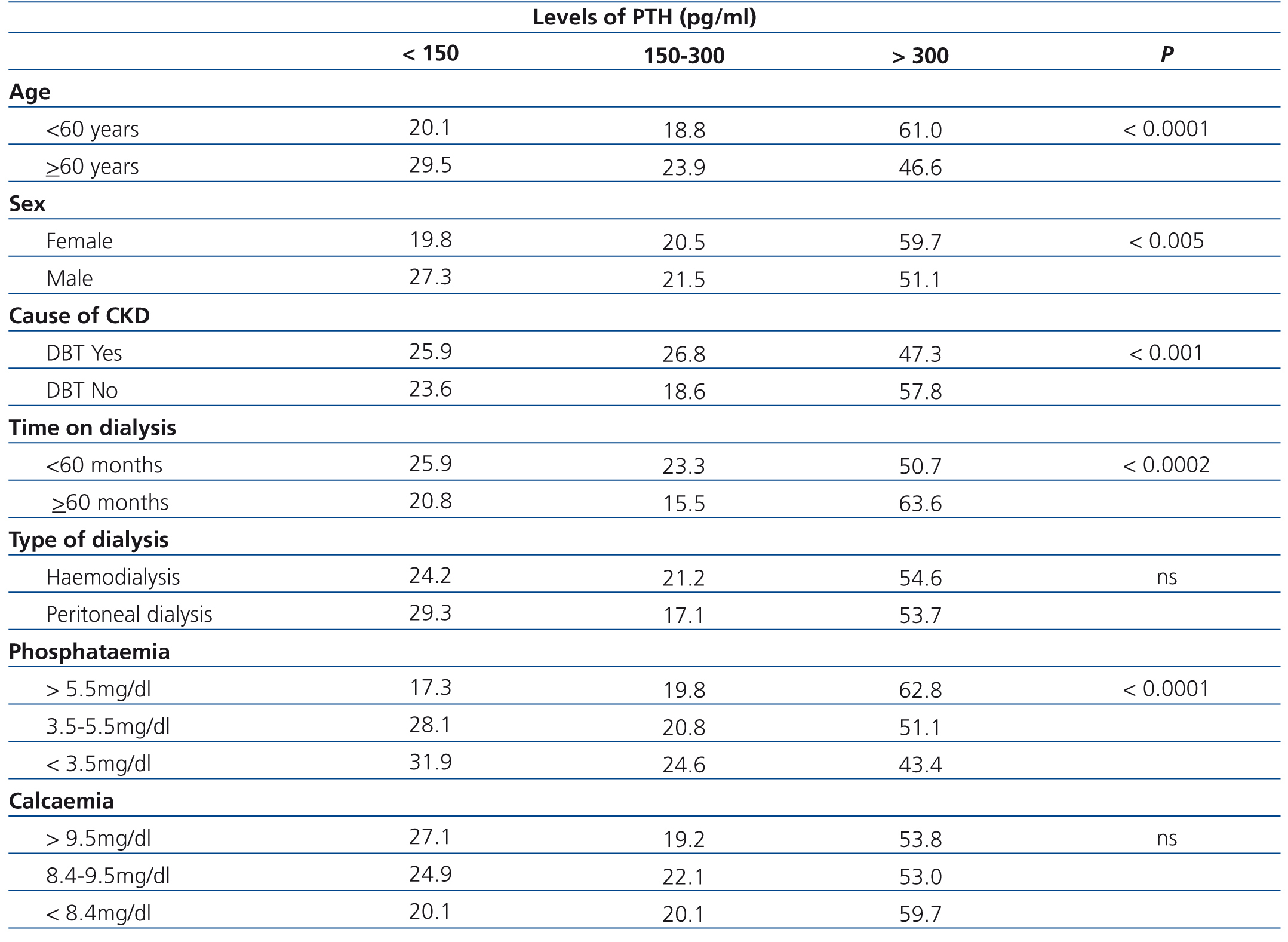
There was a wide variation in compliance with the levels suggested by KDOQI between participating centres. Thus, the percentage of patients with serum calcium levels within the range suggested by KDOQI varied between 32.3% and 82.2%, and serum phosphorus between 33.3% and 91.3% for the centres with the lowest and highest compliance, respectively. For iPTH, the percentage of compliance per centre ranged between 9.5% and 41.3%. Likewise, there was a wide variation in the presence of patients with hyperparathyroidism, with results ranging from 23.1% to 78.2%.
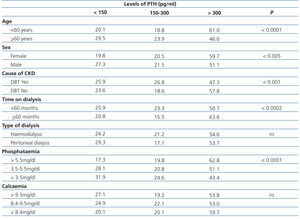
The evaluation of the different factors related to patients and forms of dialysis shows a significant association between age, sex, presence of diabetes, time on dialysis and phosphataemia with parathyroid hormone levels suggested by the KDOQI guidelines, except for calcaemia and type of dialysis, which displayed significant but not statistically significant differences (Table 3).
There were no significant differences in compliance levels of BMM markers recommended by the guidelines for patients on haemodialysis compared with those on peritoneal dialysis. Thus, the data from both types of dialysis were analysed together.
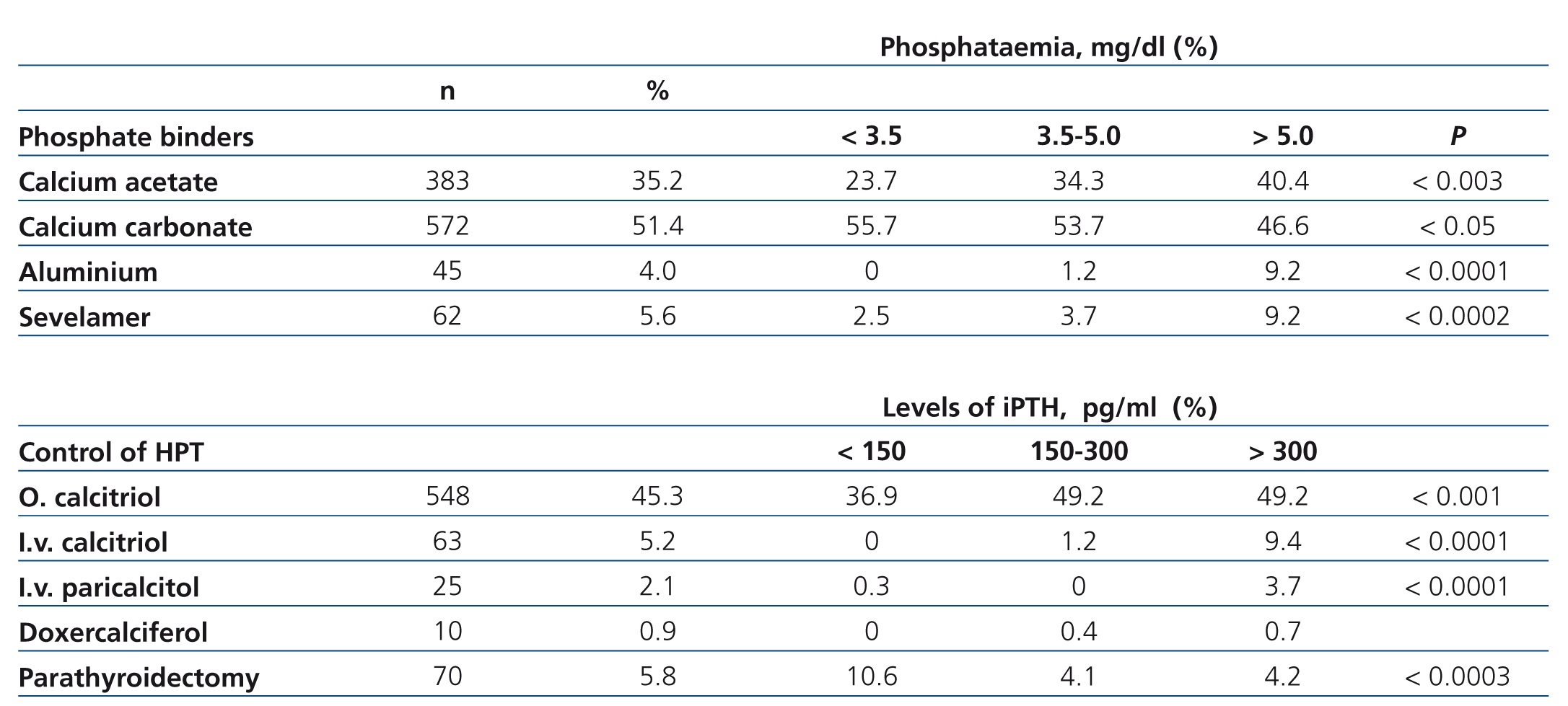
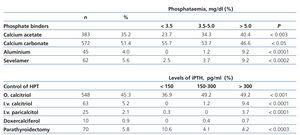
When determining the different forms of treatment used in the management of BMM disorders, we observed that 83.6% of patients used oral phosphate binders based on calcium as carbonate or acetate, while 23 patients (2.4%) used calcium acetate and carbonate in combination. 5.6% of patients were treated with sevelamer, while 4.0% used aluminium-based compounds. 79.5% of patients, despite having values consistent with hypophosphatemia (<3.5mg/dl), were using an oral phosphate binder. This proportion was 89.2% for patients with adequate phosphataemia, and 90.9% for those with hyperphosphataemia. 9.1% of patients had hyperphosphatemia but did not receive treatment with any oral phosphate binder (Table 4).
For the control of secondary hyperparathyroidism, oral or intravenous calcitriol was predominantly used (50.5%), while a small percentage of patients received intravenous paricalcitol or doxercalciferol (Table 4). 37.2% of patients with iPTH levels below 150pg/ml were treated with a vitamin D derivative. 50.8% of patients with adequate iPTH also received this treatment, as well as 62.7% of patients with iPTH above 300pg/ml and 63.9% of patients with iPTH above 600pg/ml. Levels of iPTH above 600pg/ml were detected in 26.1% of patients who did not receive any medication to control it.
The percentage of patients who did not comply with any of the normal criteria suggested by KDOQI guidelines for the main BMM parameters in Argentina and in DOPPS II was 9.5% and 17.8%, respectively, while the percentage of those who complied with the four criteria simultaneously was 5.9% and 5.5%, respectively. The importance of these data is that, regardless of the population studied, there is a marked difficulty to comply with the criteria suggested by the KDOQI guidelines. Thus, data from Argentina show that one in 10 patients did not comply with any of the standard criteria, while only one in 20 patients complied with the four criteria simultaneously. Although there were slight differences, DOPPS I and II showed similar results.
DISCUSSION
This study provides important information about the prevalence of BMM disorders and how they are managed, in a broad and representative dialysis population in Argentina. According to data from this population, patients with secondary hyperparathyroidism are prevalent and as such there is a marked difference with that found in other large studies carried out on dialysis populations in Europe, the United States and Japan such as DOPPS, in which an inverse relationship was observed in the proportion of patients with hyperparathyroidism to that of Argentina.11,12 Our study also reveals that, despite the availability of new drugs for the control of hyperphosphatemia and secondary hyperparathyroidism in Argentina, the same drugs that were available decades ago are still predominantly being used.
Bone histological forms described and their diagnostic and treatment modalities have been modified over time due to changes in the characteristics of the dialysis population and a greater knowledge and awareness of the consequences of these disorders.17-19
Thus, between the 1970’s and 1980’s, the low remodelling forms associated with bone aluminium overload were prevalent, while between the 1990’s and the new century, those with high remodelling were prevalent with differences in accordance with the regions involved.4,5,20
Practically the only data published to date on forms of BMM disorders prevalent in the dialysis population in Argentina come from our group through the study of bone biopsies taken during the 1990’s and at the beginning of the new century. They show hyperparathyroidism (37.8%) and mixed bone disease (27%) as the prevalent forms in Argentina.7 Similar results were found in the dialysis population in Uruguay and Brazil. About one third of patients displayed significant deposits of aluminium in bone with low remodelling forms.13
Towards the end of the 1990’s and to date, most epidemiological reports have been based on clinical and biochemical data of BMM markers,21-23 probably because of the growing evidence of a close association between changes in BMM biochemical markers and the presence of cardiovascular calcifications and mortality.24-27 Results of the CORES study were recently published with retrospective data from the database of patients with CKD on dialysis at Fresenius Medical Care centres in six Latin American countries. In this study, which was carried out to determine mortality in relation to BMM markers, the percentage of patients with iPTH levels above 300pg/ml was 30.9%, those within the range suggested by KDOQI was 26.2%, and those below 150pg/ml was 42.8%.28
One of the largest and most representative observational studies carried out on patients on dialysis is the DOPPS12 multinational study. This study described the state of BMM disorders and their management in five European countries, the United States and Japan, between 1996 and 2001 (DOPPS I) and 2002 and 2004 (DOPPS II).11,12 Taking as optimal iPTH values those suggested by KDOQI (150 to 300pg/ml), 51.1% had values below 150pg/ml, while only 26.7% had levels of iPTH >300pg/ml. This study demonstrated that, depending on the country studied, there were differences in the prevalence of different BMM disorders. Thus, the percentages of patients with low or high PTH were 58.6% and 19.0% for Japan, 50.1% and 26.9% for European countries and 48.2% and 30.3% for the United States, respectively.8 The factors related to the probability of having hyperparathyroidism were young age, being female, having lower haemoglobin and Kt/V, absence of diabetes, human immunodeficiency virus and receiving dialysis treatment in Japan. The percentage of patients outside the range suggested for serum phosphorus (3.5 to 5.5mg/dl) was 51.6% and 7.6% for hyper and hypophosphatemia, respectively, whereas for suggested levels of calcaemia (8.4 to 9.5mg/dl) it was 50.2% and 9.3% for hyper and hypocalcaemia, respectively.8
Our study shows the biochemical data and the type of management of BMM disorders for the 1210 patients from 25 dialysis centres in the central region of Argentina, which represent 4.7% of the total population on dialysis in the country to 2010 (Table 1). Unlike that observed in DOPPS I and II, data from Argentina show a greater proportion of patients with hyperparathyroidism, including a large number of patients with severely high values over 1000pg/ml (Figure 1). For a projection of these data in accordance with the prevalence of the dialysis population in Argentina to 2010, we would expect about 7300 patients to have an iPTH value above 600pg/ml and about 3500 patients to have an iPTH value greater than 1000pg/ml. According to the data of our study, only 24.4% of patients had values below 150pg/ml.
Furthermore, our study shows that a large number of patients have levels of serum calcium and phosphorus outside those suggested by the KDOQI guidelines.14 Mean levels of iPTH were markedly above the upper limit suggested by KDOQI of 300pg/ml (Table 2), with a significant association being found with age, sex, the presence of diabetes, time on dialysis and the different levels of phosphataemia (Table 3).
Data from both the DOPPS and Argentinian studies reflect the difficulties in keeping patients within the range suggested for BMM markers. This difficulty is even more pronounced for iPTH, as only a small percentage of patients in both studies (just over 20%) were able to achieve the proper range. For calcaemia and phosphataemia, about half of the patients were within the expected values; there were no differences between the two studies in the percentage of patients who met KDOQI criteria (Table 2). Such is the difficulty of achieving the values suggested by KDOQI that in the DOPPS study, 1 in 5 patients and in the Argentinian study, 1 in 10 patients did not comply with KDOQI criteria for any of the BMM markers. By contrast, when we studied the percentage of patients who had the four BMM within the target range, only approximately 1 in 20 patients in both studies achieved this target.
Our study does not allow evaluation of the factors for which the Argentinian population has a different percentage of patients with hyperparathyroidism, compared with patients in the DOPPS study. While some characteristics of both populations were different (age 60.5 versus 55.3 years, time on dialysis 37.2 versus 47.5 months, 57.4% male vs. 60.8% female, 19.2% black vs. 0% black, diabetes as a cause of CKD 31.6% vs. 29.1%, peritoneal dialysis patients 0% vs. 3.3%, between DOPPS and the Argentinian study, respectively), they do not seem to be strong enough to justify these findings (Table 1 and Figure 1). Perhaps one explanation may be found in the type of treatment used for managing BMM disorders and in particular for controlling hyperphosphataemia and secondary hyperparathyroidism.
The treatment of BMM disorders requires an adequate combination of effective dialysis therapy, dietary adjustments, proper use of medications and patient adherence to therapies implemented.29,30
Our data show that although a large proportion of the patients used an oral phosphate binder, most received calcium-based compounds and only a small number were treated with sevelamer or aluminium (Table 4). Nearly a decade ago, 81% of patients in the DOPPS study used oral phosphate binders, of which 53% were calcium carbonate, 26% were calcium acetate, 6% were aluminium salts and 1.5% was magnesium, and only 0.1% received sevelamer at the time.8 The most striking factor in both studies is that a high proportion of patients with low phosphataemia received a phosphate binder (77% in the DOPPS study and 79.4% in the Argentinian study) (Table 4).8
With respect to treatment used to control hyperparathyroidism in the case of the Argentinian study, most of the patients were treated with oral or intravenous calcitriol, while only a small proportion were treated with a derivative of vitamin D (Table 4). At the time this survey was carried out, cinacalcet was in the process of being approved by the Argentinian authorities. Despite the high percentage of patients with hyperparathyroidism, the number of patients with a parathyroidectomy was low (Table 4). The DOPPS study showed that 52.2% of patients received vitamin D, without its form being specified.8 As with phosphate management, a high percentage of patients with PTH levels below those recommended by KDOQI guidelines received a form of vitamin D (46% of patients in the DOPPS study and 37.2% in the Argentinian study) (Table 4).8 37% of patients in the Argentinian study did not receive treatment to control high levels of PTH, while 4.2% of patients maintained PTH values above 300pg/ml despite receiving a parathyroidectomy (Table 4).
This study shows that towards the end of the first decade of the century, mainly calcium-based phosphate binders were still used in Argentina and to control hyperparathyroidism, calcitriol continued to be used; both of these medications appeared in the 1980’s and 1990’s. This shows a low use of new treatments available.
The main limitation of the study is its cross-sectional and observational nature. Data were collected through a survey responded to voluntarily and without financial compensation for researchers. Since each centre used its own laboratory for monthly measurements, we did not have the means to perform quality control. Since we used a survey, we had to limit the number of data required from researchers with the aim of achieving higher accuracy and reliability in responses. This allowed us to meet our objective of describing the reality of BMM disorders in Argentina, but we were limited in terms of finding the causes.
To our knowledge, these are the first data published on the forms of presentation of BMM disorders in Argentina through the study of BMM biochemical markers. This study provides important information about the reality of this disorder in the dialysis population in the region.
Acknowledgements
We wish to thank nephrologists who contributed patient data and those of their respective dialysis centres.
Participating dialysis centres: Aterym Alta Gracia, Córdoba: Drs Sergio Boni and Carolina Nadaya. Centro Caroya Renal Privado, Córdoba: Drs María del Carmen Pievaroli and Silvana Lisiardi. Centro de Diálisis Diaverum SA, Bariloche: Drs Nelson Junqueras and Eduardo de Orta. Centro de Enfermedades Renales, Catamarca: Drs Gregorio Villafañez and Angélica Naudi. Centros Privados de Hemodiálisis SRL, San Francisco: Drs Gustavo Díaz Cornejo and Maximiliano Tejerina. CEPER, San Francisco, Córdoba: Drs Carlos A. Castellano and María Luisa Favaro. CIPERCA SRL, Catamarca: Drs Segundo Fernández and Cinthia Fernández. INER Siglo XXI, Paraná, Entre Ríos: Drs Ramón Giacchi and Bruno Obaid. Unidad de Diálisis del Hospital del Centenario, Rosario, Santa Fe: Drs Griselda Nicola and Claudio Mascheroni. Unidad de Diálisis del Hospital Privado-Centro Médico de Córdoba: Drs Walter Douthat and Javier de Arteaga. Instituto de Nefrología y Hemodiálisis Kolff SRL, Villa María, Córdoba: Drs María Gabriela Bergamín and Amadeo Ancarani. Instituto Integral de Nefrología San Lorenzo, Santa Fe: Drs Alberto Alles and María Laura Benítez. Nefro One Valles Calchaquíes, Salta: Drs Germán Gálvez and Mario Espeche. Salud Renal SRL, San Luis: Drs Paula Arenas and Julio Bittar. SEPRINE La Carlota, Córdoba: Dr Darío Rubén Lladser. Servicio de Nefrología y Hemodiálisis de Bell Ville, Córdoba: Dr Luis E. Rivera. Servicio de Nefrología Privado Mayo SRL: Dr Héctor Enrique Aliciardi. SUA SRL, Jesús María, Córdoba: Dr M. Susana González. Unidad de Hemodiálisis, Instituto Médico Río IV, Córdoba: Drs Carlos E. Fragueiro and Eugenio Quero. Unidad de Hemodiálisis de San Pedro, Buenos Aires: Drs Mariela Fernández and Fabio Acosta. Unidad Renal Chilecito, La Rioja: Dr Daniel A. González. Unidad Renal Corrientes: Drs Juan José Di Bernardo and Luis R. Urtiaga. Unidad Renal General Deheza SRL, Córdoba: Drs Juan C. Abascal and Fernando Massei. Unidad Renal Justiniano Posse Privado: Dr Fernando Massei. Unidad Renal Río IV, Córdoba: Drs Juan Carlos Abascal and Cecilia Grahovac.
Conflicts of interest
The authors report potential conflicts of interest.
Grants: Drs M.ª Alejandra Guzmán, Leandro Berenguer and Mauro Castellano receive grants from the Fundacion Nefrológica de Córdoba.
Lecture fees: Dr Walter G. Douthat receives lecture fees from Laboratorios Amgen.
Board membership: Dr Walter G. Douthat is President elect of the Sociedad Latinoamericana de Nefrología.
Travel bursary or travel expenses: Dr Walter G. Douthat received a travel grant from Laboratorios Raffo, Argentina.
Table 1. Characteristics of participating dialysis centres and patients
Table 2. Levels of mineral metabolism markers in dialysis patients in Argentina and the levels suggested by the bone and mineral metabolism guidelines
Table 3. Percentage distribution of patients according to values suggested by the KDOQI14 guidelines for the parathyroid hormone, in accordance with various factors that may affect bone and mineral metabolism
Table 4. Number and percentage of patients receiving single or combined treatment for the control of hyperphosphataemia or secondary hyperparathyroidism
Figure 1. Percentage distribution of patients in accordance with mineral metabolism marker levels distributed in accordance with values expected by K/DOQI and K/DIGO14,15 guidelines.