Objetivos: Valorar el grado de cumplimiento y la variabilidad de los parámetros del metabolismo mineral a lo largo del tiempo en una muestra de pacientes en hemodiálisis según los distintos rangos propuestos (Guía KDIGO/Recomendaciones S.E.N.) tanto a nivel de grupo como individual de forma continuada. Material y métodos: Recogimos cuatrimestralmente los datos de calcio, fósforo, hormona paratiroidea (PTH) y de tratamiento en una muestra de 44 pacientes seguidos de forma continuada durante 32 meses. Establecimos los porcentajes de pacientes que cumplían los objetivos propuestos para cada parámetro en ambos rangos: óptimo (KDIGO) y aceptable (S.E.N.) en cada control y el porcentaje que cumplían individualmente los objetivos en al menos un 75 % de las determinaciones. Resultados: El cumplimiento del objetivo utilizando el rango óptimo mejoró, aunque en la PTH no superó el 50 %. Utilizando el rango aceptable, el objetivo se alcanzaba en los tres parámetros por encima del 70 % y más del 50 % de los pacientes cumplían los tres simultáneamente, mientras que utilizando el rango óptimo nunca se alcanzó el 30 %. A nivel individual el cumplimiento del rango óptimo se alcanzó de forma continuada en el 52,3 % (calcio), 45,5% (fósforo) y solo un paciente en la PTH, mientras que utilizando el rango aceptable el cumplimiento se alcanzó en el 84,1 % (calcio) y el 70,5 % (fósforo y PTH). Conclusiones: El uso de criterios menos exigentes que los KDIGO en los objetivos de calcio, fósforo y PTH permite mantener de forma continuada a los pacientes en rangos adecuados con menor intervencionismo y menor variabilidad individual.
Objective: To assess the level of compliance and variability of mineral metabolism parameters over time in a sample of haemodialysis patients for the different ranges proposed (KDIGO guidelines/S.E.N recommendations) in both groups and individuals continuously. Material and method: Every four months, we collected data on calcium, phosphorus, PTH and treatment in a sample of 44 patients followed up continuously for 32 months. We established the percentages of patients who complied with the objectives set for each parameter in both ranges: optimal (KDIGO) and acceptable (S.E.N.) in each control and the percentage that individually complied with the objectives in at least 75% of the determinations. Results: Compliance with the objective using the optimal range improved, although PTH did not exceed 50%. Using the acceptable range, the objective was achieved in the three parameters in over 70% and over 50% of patients achieved the three simultaneously while using the optimal range, 30% was never achieved. Individually, compliance with the optimal range was continuously achieved in 52.3% (calcium), 45.5% (phosphorus) and in only one patient in PTH, while when using the acceptable range, compliance was achieved in 84.1% (calcium) and 70.5% (phosphorus and PTH). Conclusions: The use of less stringent criteria than the KDIGO guidelines in calcium, phosphorus and PTH objectives allows patients to remain continuously within appropriate ranges with less intervention and less individual variability.
INTRODUCTION
Recommendations for the treatment of mineral bone metabolism disorders have been changing over the past few years as more knowledge has been acquired and more has been understood about it. In 2009, the KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention and Treatment of Chronic Kidney Disease Mineral and Bone Disorder,1 was published, in which there was an update of the targets to achieve in relation to calcium, phosphorus and the parathyroid hormone (PTH) levels in patients on haemodialysis (HD), with more “stringent” ranges being established than those included in the 2003 KDOQI guidelines.2 For phosphorus, the range should be between 2.5 and 4.5mg/dl (2D), for calcium it should be between 8.4 and 9.5mg/dl (2D) and for PTH, between 2 and 9 times the normal limit of laboratory tests (2C). In 2011, the Spanish Society of Nephrology (S.E.N.) also updated its recommendations for the management of these disorders in order to assist nephrologists in their management and treatment and although they advise keeping calcium (2D) and phosphorus levels (2C) in the normal laboratory range with values for PTH between 150 and 300pg/ml (2B), they permit less stringent ranges with “tolerance” for values up to 10mg/dl for calcium and up to 5mg/dl for phosphorus (2B), and values less than 100pg/ml and more than 500pg/ml should be avoided for PTH.3
Achieving the highest possible number of patients within the limits set out in these guidelines for phosphorus, calcium and PTH control, in spite of the new recommendations and therapeutic advances, continues to be a challenge for professionals due to its difficulty, with the percentage of patients controlled and efforts varying in accordance with the flexibility in the target range that we set.
The aim of our study was to analyse, in a stable group of HD patients over 32 months, the percentage that achieved the targets set for the following criteria: the narrow range (that we will call optimal) of the KDIGO guidelines and the tolerance range (that we will call acceptable) of the S.E.N. recommendations (Table 1), and the degree of compliance, in each of the patients, with the three parameters throughout the follow-up period, which provided us with a view of individual variability, an aspect that is less assessed in the literature.
MATERIAL AND METHOD
We carried out a retrospective study on all patients in the HD unit of the Hospital Virgen de los Lirios of Alcoy, which has a catchment area of 140 000 inhabitants, who complied with the following criteria in October 2012: inclusion in an HD programme for at least six months before February 2010 and having received treatment with this modality (HD) uninterrupted during the study period (32 months).
The monitoring and management of patients’ mineral metabolism was performed by testing calcium and phosphorus monthly and PTH bimonthly (electrochemiluminescence immunoassay) measured by the Cobas® autoanalyzer (Roche Diagnostics®). With the results obtained, we modified the treatment regimen in accordance with the S.E.N.3 recommendations: dietary intervention, the use and modification of binder doses (as a general rule, we used calcium acetate as the first binder option and gradually increased the doses in order to achieve phosphorus of ≤4.5mg/dl; when the patient reached a calcium level of 9.5mg/dl, a calcium binder was added as needed), with doses of paricalcitol and cinacalcet being introduced and adjusted, as well as the calcium concentration of the dialysis bath being modified.
For the analysis over the 32 month period, we collected every four months the laboratory data for calcium (corrected if serum albumin was below 3.5g/dl), phosphorus, PTH and Kt/V and those for the treatment received at the time of the test: intravenous (i.v.) paricalcitol dose, cinacalcet, type and dose of binders (calcium acetate associated or not with magnesium hydroxide, calcium carbonate, aluminium hydroxide, sevelamer and lanthanum) and calcium concentration of the dialysis bath.
The data were collected on a spreadsheet and transferred for statistical analysis to the G-Stat 2.0 software. The data were expressed as a mean ± standard deviation or median (p25-p75) in accordance with their distribution. We assessed the existence of differences in laboratory parameters in the different controls through tests for paired data: one-way ANOVA (calcium, phosphorus. Kt/V) or Friedman’s test (PTH) in accordance with their distribution; drug doses (paricalcitol, cinacalcet and binders) were analysed using the Kruskal Wallis and Mann Whitney tests. The comparison between proportions was carried out using the χ2 test for linear trend for 2 x n groups and McNemar’s test for 2 x 2 groups. Statistical significance level was established for P values <.05.
We established two ranges of targets for laboratory parameters, on the basis of the KDIGO and S.E.N. guidelines, which we called the “optimal range” and “acceptable range”, respectively. We obtained the percentage of patients who met the targets in the two ranges proposed for each parameter (calcium, phosphorus and PTH) in each control and lastly, the percentage of patients who individually met the targets in at least 75% of the tests (7 of 9 controls), as well as the number of indicators in target values in at least 75% of the tests.
RESULTS
Patients
In the study, we included 44 patients: 29 males (66%) and 15 females (44%) of a mean age of 70±12 and with a period on HD before the start of the study of 41 (31-96) months, regardless of the time on other renal replacement therapy modalities or previous periods on HD. Prior to HD, 8 patients (18%) had received a kidney transplant and 4 (9%) had been treated with peritoneal dialysis. A parathyroidectomy was performed on 4 patients (9%). Thirty-seven (84%) received HD through a native arteriovenous fistula, 6 (14%) via a tunnelled catheter and only 1 (2%) had a polytetrafluoroethylene (PTFE) prosthesis. The mean dialysis dose (mono-compartment Daugirdas Kt/V) increased progressively in each control from 1.57±0.26 at the start to 1.72±0.22 at the end of the study (P<.001).
Progression of the laboratory and treatment parameters
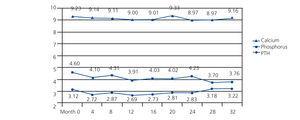
There was a continuous progressive significant decrease in mean phosphorus values from 4.60±1.26 at baseline to 3.76±1.92mg/dl at month 32 (P<.001) with a maximum value of 9.33mg/dl and a minimum value of 8.97mg/dl, but without a defined trend throughout the study (baseline 9.23±0.54 compared with 9.16±0.50mg/dl after 32 months [not significant, ns]). In the case of PTH, there were no significant variations in its values from 312 (152-369) to 322 (127-411) pg/ml (ns) at the end of the study (Figure 1).
The frequency of i.v. paricalcitol use increased over the 32 months from 38.6% (17/44 patients) to 63.6% (28/44 patients) (P<.05). The dose used at baseline was 8.3±5.4µg/week; however after 4 months the dose had decreased to 4.4±3.0µg/week and from then on, it ranged between 5.0 and 3.6µg/week over the whole study. In the first 12 months, the maximum dose of paricalcitol was 15µg/week and from then onwards, the dose was no greater than 9µg/week in any patient. The percentage of cinacalcet use progressively increased from 25.0% (11/44 patients) to 40.9% (18/44) (P<.05), with a mean dose that decreased from 325±250mg/week to 198±136mg/week (ns, P=.07) (Figure 2).
The most commonly used binder was calcium acetate, associated or not with magnesium carbonate, which between 75.0% and 54.5% patients received in accordance with the study period, with a mean dose between 2.9 and 2.4g/day. The most commonly used non-calcium binder was sevelamer (between 38.6% and 25.0%), with a mean dose between 5.1 and 3.5g/day. The use of aluminium hydroxide, calcium carbonate and lanthanum was much lower and always below 10% from months 4, 8 and 12 respectively. In no case was there an upward trend in the use of binders and there was a downward trend in the use of aluminium hydroxide (P<.001) and lanthanum (P<.005) (Table 2).
With respect to the concentration of calcium in the bath, the most common was 3.0mEq/l, between 61.4% (27/44) in months 4 and 8 and 79.6% (35/44) at the end of the study, while the calcium concentration of 2.5mEq/l was used in 31.8% at the start of the study, with its use reducing to 18.2% at the end. These variations were not significant in any of the two cases. The bath with calcium of 3.5mEq/l was not used initially, but its use was required in exceptional cases to compensate hypocalcaemia induced by the use of cinacalcet, and at the end of the study, it was only used in one patient.
Progression of the degree of compliance with the indicator targets in the group (Figure 3A, Figure 3B and Figure 3C)
The percentage of patients who maintained the optimal calcium range (8.4-9.5mg/dl) varied between 65.9% at the start and 77.3% at months 12 and 16, while those who maintained the acceptable range (8.4-10mg/dl) remained above 80% for the whole study, reaching 90.9% at the end. In no case were there significant differences. The percentage of patients with calcium >10mg/dl was not above 6.8% in any control, and as such, the non-compliance with this indicator target was mainly due to levels below the proposed range.
In the case of phosphorus, there was an increase in the percentage of patients within the optimal range (2.5-4.5mg/dl) from 52.3% at baseline to 79.6% after 32 months (P<.005), while the increase in the number of patients within the acceptable range (2.5-5mg/dl) from 70.5% to 88.6% was not significant. The failure to comply with the indicator target was generally due to phosphorus >5mg/dl, but in the latter months (months 28 and 32) hypophosphataemia (13.6% in month 28) was more common.
Lastly, there was no significant trend in the compliance with PTH over the study period. The percentage of patients within an optimal range (150-300pg/ml) ranged from 18.2% (month 28) to 45.5% (months 20 and 24). This trend was not significant for compliance with the acceptable range, but in this case, the percentage was always above 70%, except in month 28 (56.8%). Failure to comply with the indicator was generally greater than the proposed range.
The number of indicators within the optimal range was 0 in 15.9%, 1 in 29.5%, 2 in 36.4% and 3 in 18.2% of patients, and at the end of the study, 0 in 0%, 1 in 31.8%, 2 in 54.6% and 3 in 13.6%. In the case of the acceptable range, the objective was achieved in the three indicators in over 50% of patients, except in month 28 (40.9%).
Compliance with targets at an individual level (Figure 4)
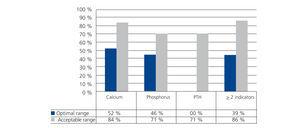
The number of patients who continuously complied with (more than 75% of the tests, at least 7 out of 9 controls) the optimal range of the indicator was, in the case of calcium, 23 (52.3%), for phosphorus it was 20 (45.5%) and in the case of PTH, it was only one patient. When the range proposed was that considered acceptable, the patient figure for continuous compliance was 37 (84.1%) for calcium, 31 (70.5%) for phosphorus and also 31 (70.5%) for PTH.
Seventeen patients (38%) complied with, in over 75% of controls, at least two of the three indicator targets within the optimal range and when the range considered was acceptable, the number of patients increased to 38 (86.4%).
DISCUSSION
From the results obtained in our study, we want to establish two lines of discussion. On the one hand, the analysis of the degree of compliance with the targets in accordance with the range (the optimal range [KDIGO guidelines] or the acceptable range [the S.E.N. recommendations]) and on the other hand, the analysis of the objectives on an individual level (variability).
When we review the results of some of many studies in the literature, we find data that for many nephrologists who work in clinical practice on a daily basis, are not easy to interpret. On the one hand, we are encouraged to obtain the highest number possible of patients within the targets set in order to decrease morbidity and mortality, but, on the other hand, we do not clearly find from which ranges the benefit that we are seeking is evident.
In general, mortality risk appears to be linked to high levels of calcium, phosphorus and PTH, both in studies that use baseline tests and in time-dependent studies.4-7 Low calcium and phosphorus levels are also associated with an increased risk of mortality,5-7 and there is some discrepancy regarding low levels of PTH, with there being an increased risk in some studies5,7 but not in others.4-6 In these studies, it is important to note that there are differences in the design, targets set, the therapeutic procedures followed by patients and the statistical analyses carried out.
Achieving mean phosphorus and calcium values within the optimal range proposed by the KDIGO guidelines was possible from the first control. The progressive and sustained decrease in phosphorus values in our patients who began with mean values of 4.6mg/dl (above the range recommended by the KDIGO guidelines), took place fundamentally due to an adequate therapeutic management of paricalcitol and cinacalcet. There was a progressive increase in the number of patients who received paricalcitol and cinacalcet over the study, although, from month 12, high doses of paricalcitol were avoided, with there being a tendency to use progressively lower doses of cinacalcet (in some patients the interval between doses administered even increased to over 24 hours), with the aim of maintaining the balance between the decrease in levels of calcium and phosphorus secondary to cinacalcet with its increases in relation to the use of paricalcitol. This reduction of the mean phosphorus values was achieved without increasing the number or the dose of binders. We do not know how the increase in the dialysis dose received may have affected phosphorus values. There were significant differences in the mean values of calcium over the study period in relation to the adjustments in the doses of drugs and in calcium concentration in the dialysis bath (the concentration most used was 3mEq/l), but with mean values always in adequate ranges in accordance with the KDIGO guideline recommendations and with a low frequency of hypercalcaemia.
The same did not occur with the mean values of PTH. We think the fact that the frequency of severe hyperparathyroidism in our patients was not high, with PTH values above 369pg/ml in only 25%, was the determining factor for mean PTH values not having significantly changed, despite therapeutic changes, since we used low doses of cinacalcet and paricalcitol. In any case, central tendency value measurements such as mean or median, are of little value in patient group studies because, although they provide us with information about behaviour and can help us assess trends or progression, they do not relate much to the balance-overload potential of both elements3 and do not reflect the percentage of patients inadequately controlled and therefore liable for another therapeutic approach.8
Neither in the KDIGO guidelines or S.E.N. recommendations for the treatment of bone and mineral metabolism disorders were any targets established in terms of the percentage of patients that should be within the limits proposed. As such, in 2007 the S.E.N. quality group published a clinical monitoring system for HD patients that included the development of a set of indicators and a percentage was established to be achieved for phosphorus and PTH, in order, through its knowledge, to improve the quality of healthcare and reduce the variability of our daily clinical practice.9
When we analysed our group indicator results on the basis of the optimal targets of the KDIGO guidelines, we noted a significant improvement in the percentage of patients who achieved them, with the percentage increasing from 65.9% to 77.3% for calcium, 52.3% to 79.6% for phosphorus, while for PTH values there was no significant trend throughout the study, with the figure varying between 18.2% and 45.5%.
These results are in line with others reported in the literature in which, since the introduction of cinacalcet, paricalcitol and new non-calcium binders, the percentage of patients achieving the targets set by the guidelines increased both for calcium and phosphorus and for PTH, but with variable percentages, fundamentally in the analysis of PTH behaviour and also depending on the characteristics of each study.10,18
However, if we take as reference values for dialysis patients those recognised as acceptable by the S.E.N. 2011 recommendations, which allows us to take wider ranges (calcium: up to 10mg/dl, phosphorus: up to 5mg/dl and PTH between 100 and 500pg/ml),3 we note a clear improvement in the percentage of patients that achieved the target, with 90.9% being attained for calcium, 88.6% for phosphorus and more than 70% for PTH.
At the end of the study, only 13.6% of patients had the three indicators within the range of the KDIGO guidelines, while, for the S.E.N. recommendations range, the three parameters were maintained within the target in over 50% of patients, except in one control.
From studies in the literature, we learned that keeping patients within the ranges proposed by the guidelines reduces the risk of mortality, but we are also aware that there are difficulties in defining the optimal range that must be achieved in HD patients, except for extreme values.19
Therefore, the question that we are considering, if we accept the slightly wider ranges proposed by the S.E.N., which without doubt allow a higher percentage of patients to achieve the objectives, is whether it makes sense to increase our effort to try and improve them, fundamentally because the difficulty is in PTH control.
Achieving a higher percentage of patients with PTH within narrow ranges (150-300), when the percentages of calcium and phosphorus are above 85%, seems complicated and we believe that it requires a delicate balance between higher doses of cinacalcet, adjustments of vitamin D or paricalcitol, as well as of the other participating factors. Increasing, fundamentally, the use of cinacalcet and/or paricalcitol to improve the control of PTH (values within the range proposed by the KDIGO guidelines) without doubt would have made maintaining calcium and phosphorus within the proposed ranges more difficult, once an increased percentage of patients with both levels well controlled had been achieved.
With respect to the analysis of target compliance for individuals (variability), some studies have highlighted increased mortality in HD patients in relation to the variability of haemoglobin with there being some limitations in its interpretation deriving from the definition of the concept, discrimination between its frequency or annual variation and the response to erythropoietic agents.20-22 Unlike for anaemia, there are few references when we analyse the concept of variability in relation to the parameters of mineral metabolism.5,23
We can, as we have seen, improve measures and achieve high percentages of patients within the ranges that the guidelines propose, but it is surprising to see their great variability throughout the study. Practically less than 50% of patients continuously meet the optimal range of the indicator (over 75% of tests within the latter) for calcium and phosphorus, and only one patient for PTH. These percentages logically improve (70%) when we transfer the targets of the KDIGO guidelines to the wider targets of the S.E.N. guidelines, including PTH.
Some evidence on the importance of knowing more about variability derive from studies in which it was observed that an excessive drop or increase in serum calcium greater than 0.6mg/dl in six months was associated with an increased risk of death in patients with baseline levels within the ranges of the KDOQI guidelines, something that was also observed with excessive changes in phosphorus and the calcium-phosphorus product.5 Also in another study, maintaining the highest possible number of measurements of calcium, phosphorus and PTH within the ranges of the KDOQI guidelines was associated with a significant decrease in mortality.17
In an attempt to decrease this variability, we want to insist on the convenience of using slightly wider ranges, such as the S.E.N. proposes, in the control of calcium, phosphorus and PTH. Making greater therapeutic efforts to attempt to achieve a greater percentage of patients with PTH in a narrow range without doubt involves greater therapeutic intervention and when there are many interrelated variables, there is inevitably greater variability, something that was already observed in the treatment of anaemia.24
Despite the obvious limitations of our study (retrospective on a small number of patients in a single centre), we believe that a continuous follow-up for over 30 months on 44 patients allows us to reach some conclusions or, at least, consider some point for reflection. In the follow-up of mineral metabolism disorders of HD patients, any value (mean, percentage, variability) has its importance and helps us to achieve the targets set. However, the information derived from the analysis must be carefully assessed. We have used the means to analyse trends, but they have not provided accurate information about the patients who did not meet the targets and who were therefore likely to be eligible for corrective action. The analysis of the percentages has allowed us to know which patients were outside the range, and we particularly focussed on them, with a significant improvement being achieved in the control of calcium and phosphorus (>75%) but not PTH, in accordance with the values proposed by the KDIGO, while for the acceptable ranges of the S.E.N. the percentages clearly increased for phosphorus and calcium (>85%) and PTH (>70%). The new drugs available, their proper management and an adequate selection of dialysis bath allowed us to better adjust the control of phosphorus and calcium, although the challenge remains to improve the control of PTH. This challenge should be considered in the early stages of chronic kidney disease during the pre-dialysis phase.
Lastly, we observed great variability in a large percentage of patients throughout the study when we accepted as reference values those proposed by the KDIGO guidelines. This variability decreased significantly when we accepted the wider ranges proposed by the S.E.N. recommendations with a clear improvement in the achievement of targets, including for PTH values, with over 50% of patients with all three parameters within the acceptable range throughout the whole follow-up period. Maintaining values within the adequate ranges continuously throughout follow-up in each patient is the definitive expression of correct treatment.
As the levels of evidence in targets to be achieved increase in mineral metabolism disorders in HD patients, as professionals, we should increase our efforts. In the meantime, using wider ranges, that are currently accepted, helps us to achieve our targets, decreases variability, avoids iatrogenesis and reduces costs.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Progression of biochemical parameters
Figure 2. Percentage of patients treated with paricalcitol and cinacalcet and dose in the patients
Figure 3. Degrees of compliance
Figure 4. Percentage of patients that complied with the target for each indicator in over 75% of the controls (at least 7 out of 9 tests)
Table 1. Targets for laboratory parameters
Table 2. Progression in the use of binders: number and percentage of patients treated and the mean dose used