To the Editor:
INTRODUCTION
Renal failure is a common and serious complication of multiple myeloma (MM) that leads to a significant increase in morbidity and mortality,1 with myeloma kidney being the entity most commonly found in this type of patient. The extracorporeal clearance of light chains is considered a support for chemotherapy to decrease the risk of advanced chronic renal failure and the need for chronic renal replacement therapy. It also decreases overall mortality.2
CASE STUDY
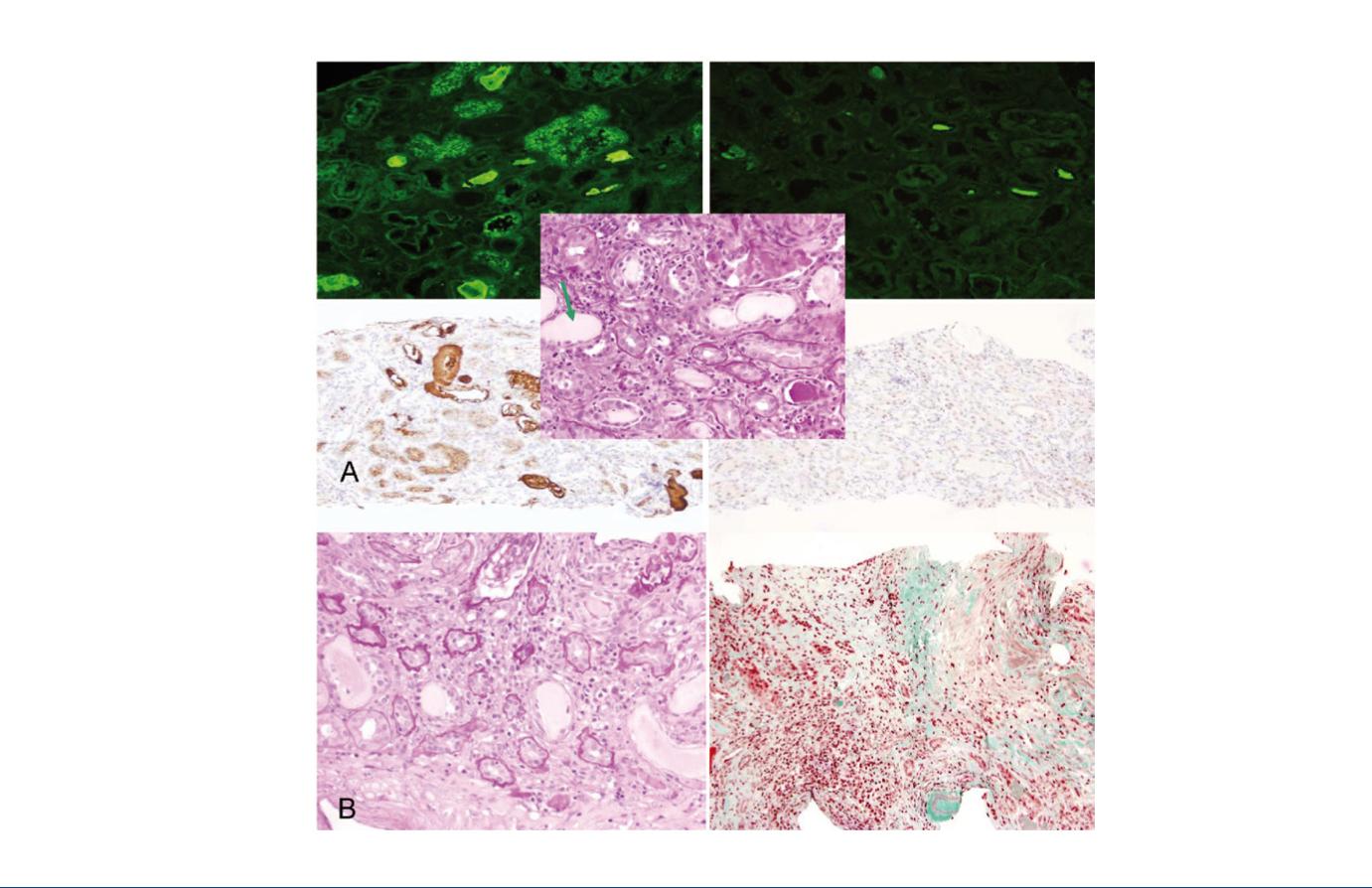
A 46-year-old patient without any relevant history, presented at the Emergency Department with pain in his left testicle irradiating to the ipsilateral flank, with no accompanying fever or urinary symptoms. The physical examination was unremarkable except for high blood pressure. The ultrasound of the kidneys and testicles-prostate was without findings. We observed rapidly progressive renal function deterioration (creatinine 9.79mg/dl, 4.85g/24 proteinuria without active sediment) and progressive anaemia (Hb 8.1g/dl, mean corpuscular volume 88fl, mean corpuscular haemoglobin 31.1, mean corpuscular haemoglobin concentration 35.4). The immunological study (anti-nuclear antibodies, anti-neutrophil cytoplasmic antibodies, anti-GBM, anti-streptolysin O, rheumatoid factor, C3-4), viral serology and tumour markers were normal. Protein electrophoresis-immunofixation with immunoglobulin (Ig) A-kappa monoclonal band. IgG 317, IgA 1446, IgM 15mg/dl, free light chains: (FLC, Free-Lite® nephelometer) kappa 4090ng/ml, lambda 1. Uric acid 10.8, LDH 269, calcium 10.2, albumin 3.3, B2 microglobulin 23,340. Liver profile, lipids and other blood count were normal. Nephropathy was anatomopathologically diagnosed due to kappa free light chain (K-FLC) casts (Figure 1A) and Durie-Salmon stage IIIB IgA-kappa MM, and the patient was treated with bortezomib-dexamethasone along with renal replacement therapy with Theralite® high cut-off filter on alternate days.
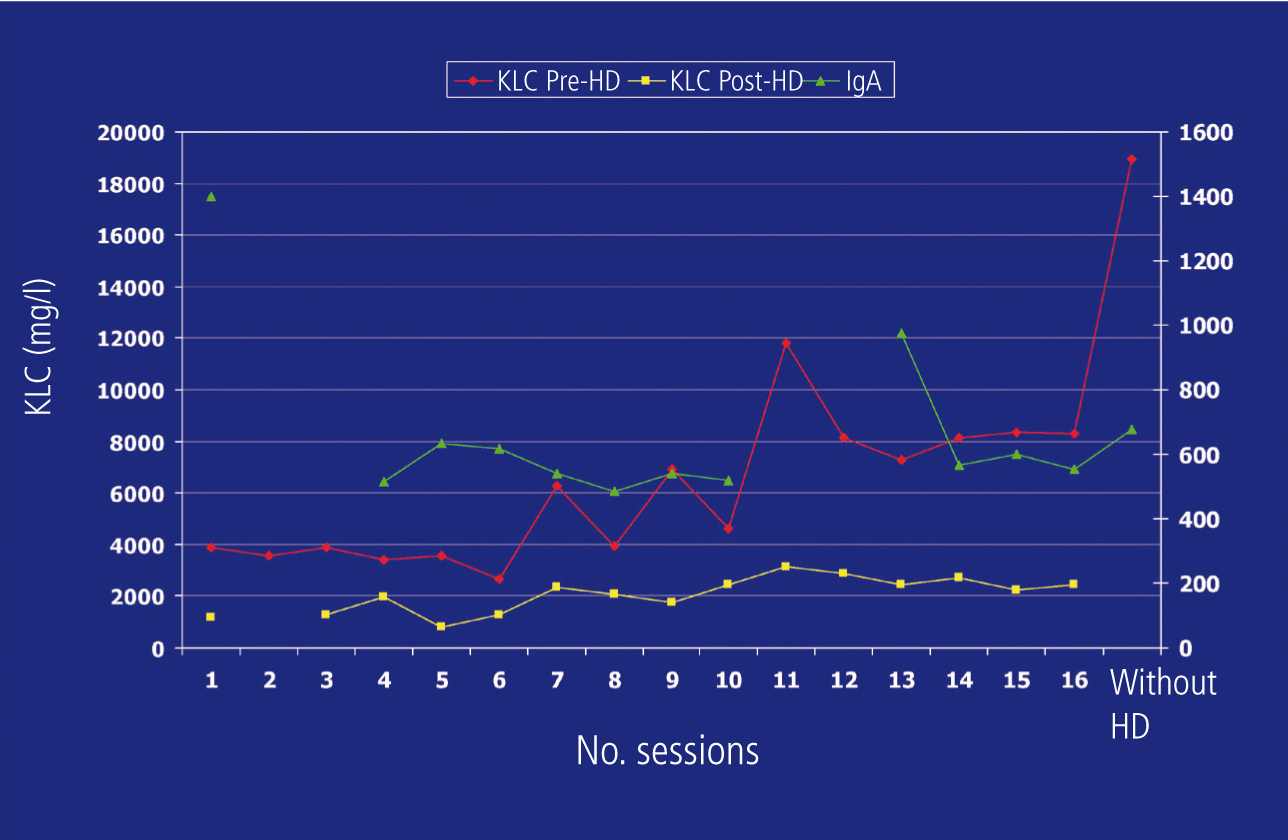
For two consecutive months, the serum IgA figure decreased progressively and significantly, which was not the case for pre-dialysis K-FLC (Figure 2), but in a multidisciplinary clinical session, it was decided to continue with the treatment established in accordance with the Haematology department protocol and re-biopsy the kidney to assess whether to continue renal replacement therapy. In the second biopsy, we observed chronic mild intensity tubulointerstitial lesions (Figure 1B). We prescribed 6 further high cut-off dialysis cycles.
Three months after diagnosis, after 4 chemotherapy cycles, bortezomib was considered to be ineffective and was replaced by lenalidomide, adjusted according to renal function. Due to financial constraints, it was impossible to continue removing FLC by renal replacement therapy (with the patient having received a total of 18 sessions) and they remained on renal replacement therapy with conventional high flow dialysis. The efficacy of lenalidomide was confirmed (Task Force, SWOG and EMBT) and the patient continues to receive this drug three years after diagnosis along with renal replacement therapy.
DISCUSSION
Renal failure in MM occurs in 12%-20% of patients, with a mean survival of less than one year in dialysis.3 Cast nephropathy is the most common cause of renal failure, with measurement of FLC in blood being a very useful diagnostic tool.4 Chemotherapy has been the key to an improved prognosis in these patients in the last decade, with light chain synthesis decreasing, causing the reduction of FLC deposits in the renal tubule, which is ultimately responsible for the nephropathy. Furthermore, extracorporeal clearance techniques that remove light chains and therefore stop their contact with the renal tubule have been tested for decades as a supportive therapy, without success in terms of renal function recovery.5 Hutchison et al.2 improved these results with a long 8-hour haemodialysis technique using high cut-off filters in which success was determined by early treatment.6
In this case, first-line chemotherapy was not effective, rendering extracorporeal clearance useless. The monitoring of kappa-FLC, not IgA, which decreased considerably from the start of treatment, seemed to indicate this therapeutic failure at all stages and the short half-life of light chains meant that monitoring them allowed us to detect changes in the tumour earlier than in whole Ig.7 As such, we observed how, after each cycle, the amount of FLC decreased significantly and increased to similar amounts in the next measurement 48 hours later, which means that the clearance filter was doing its work, which was not the case for the synthesis inhibitor treatment, i.e. chemotherapy. The contradiction between IgA levels and K-FLC levels and the non-inclusion of this FLC monitoring in the response protocols delayed the re-assessment of the patient’s treatment, which in turn interrupted their recovery from the nephropathy. So, we should perhaps consider that a same clone of B cells may secrete different quantities of whole Ig and light chains. Mead et al. found a poor correlation between levels of whole Ig and FLC in mixed myelomas.8 This, or the existence of different B cell clones, some of which segregate IgA and others, light chains, is the physiopathological explanation that we attribute to this case. As such, we rightfully believed that, although IgA levels reflected a good response to treatment, K-FLC were indicating the opposite.
Until present, FLC monitoring has not been included in standard blood protocols for response to treatment, and as such, in most centres, only whole Ig is taken into account when assessing this response. Due to the long half-life of Ig, it is monitored monthly and the response is not assessed before the fourth cycle of chemotherapy, that is, between the third and fourth month after diagnosis. The haematological argument for not advancing the abovementioned assessment is that a delay in the diagnosis of therapeutic ineffectiveness of 4-6 weeks does not really affect the final outcome, as far as the medullary response is concerned.8 as nephrologists, we insist that the situation is different whenever there is a nephropathy due to light chains, since early diagnosis-treatment is vital in this case: we can cure medullary cell dyscrasia at a later stage, but we cannot do so for renal tubular cells, as demonstrated in the second biopsy carried out in our case.
Fortunately, more and more studies advocate the prognostic importance of FLC9 and the inclusion of their monitoring in these protocols, which could even save a re-biopsy of the medulla.7-11
CONCLUSION
Early chemotherapy and clearance of free light chains is fundamental in order for treatment to be effective in myeloma kidney. The monitoring of free light chains in blood helps to assess the therapeutic response. Good coordination between Nephrology and Haematology is essential for treatment to be effective in these patients.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Renal biopsies.
Figure 2. Monitoring of kappa light chains and immunoglobulin A.