To the Editor,
Sirolimus, an immunosuppressive agent used to prevent graft rejection, has a narrow therapeutic window and high interindivual and intraindividual variability. Its concentration in blood must be monitored to prevent graft rejection and some adverse effects.1 To date, the microparticle immunoassay (MEIA, Abbott Laboratories®) on an IMx® analyser has been the most used method for measuring sirolimus concentrations in blood.2-6 However, the 2010 Abbott Laboratories® stopped marketing the reagents for this technique, replacing them with a chemiluminescent microparticle immunoassay (CMIA) on the Architect® analyser. Different immunoassays do not always yield the same results, given that techniques can have different sample pretreatments, drug metabolite cross-reactivity, or quantification limits.
The aim of our study was to compare sirolimus limits in kidney transplant patients, obtained by analysing the same blood sample with the two immunoassays (IMx® and Architect®). The sirolimus concentration analysis included the samples received at Del Mar Hospital during the first half of 2010 (period in which both reagents were available). We analysed 21 samples from 13 kidney transplant patients (10 men, age: 57.5 years [SD=12.4], post-transplant time: 5.25 years [Q1-Q3=4.13-9.44]).
Average concentrations obtained were 4.98ng/ml (SD=2.14) for IMx® and 8.37ng/ml (SD=3.01) for Architect®. The mean absolute difference between the techniques was +3.39ng/ml (SD=1.76) for Architect® compared to IMx®.
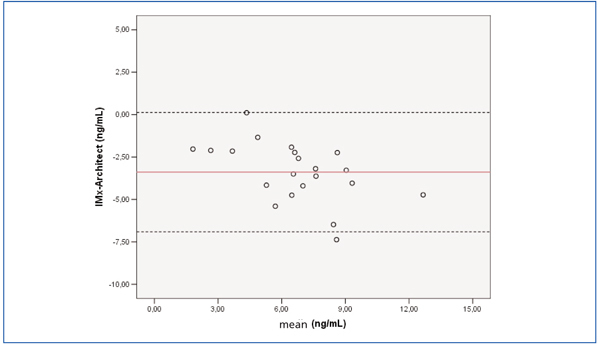
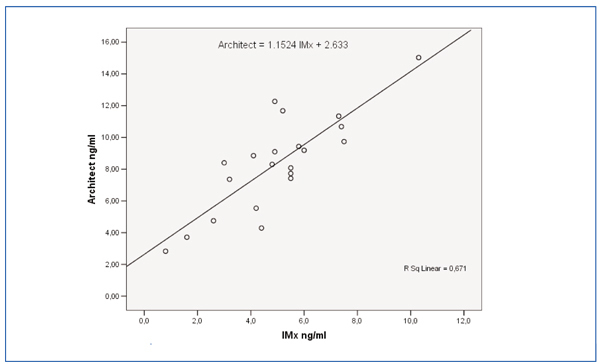
The Bland-Altman graph in Figure 1 shows the differences between the two techniques. Figure 2 shows the correlation of least squares between both techniques. The Pearson’s correlation coefficient was r=0.819.
For 13 of the 21 samples, the difference between the two techniques was more than 50%, especially for samples less than 5ng/ml (9/11 compared to 4/10; P=.080).
Two of the samples analysed by IMx® (9.5%) were below their quantification limit (QL: 2.5ng/ml), while this was not found for the Architect®-analysed samples (QL: 0.7ng/ml).
For the IMx®-analysed samples, 47.6% (10/21) were within the therapeutic window (5-15ng/ml), the remaining 52.4% (11/21) were at an infra-therapeutic level. However, of the Architect®-analysed samples, 76.2% (16/21) were within the therapeutic window, 19.0% (4/21) were at an infra-therapeutic level and 4.8% (1/21) at a supra-therapeutic level.
Various immunoassays have been developed, making immunosuppressive drug monitoring easier.7,8 Immunoassays use reagents with monoclonal antibodies against the drug analysed. Depending on the antibody’s specificity, cross-reactivity may exist with the drug’s metabolites. This cross-reactivity varies for each technique, giving rise to differences in the results from different immunoassays. This variance could cause conflict in deciding upon an immunosuppressive dose.
Our results show that Architect® shows 3ng/ml more than IMx®. Courtais et al obtained similar results with slightly lower difference (2.28±1.28ng/ml). However, only 4 out of the 53 patients studied had undergone a kidney transplant.9 Furthermore, the difference was only calculated for 51 out of the 100 samples analysed, meaning that the infra-therapeutic or the supra-therapeutic ones were not considered.
According to the HPLC data provided at that time by the United Kingdom External Quality Assessment Service (UK NEQAS) for Clinical Laboratories, IMx® presents a bias of around -10%, and Architect® of +15%-20%.10
These differences can be due to different causes. Firstly, the two techniques use different methods for extracting the drug from the protein FKBP12. Dimethyl sulfoxide (DMSO) is used in Architect® pretreatment to heat the sample so that more sirolimus can be extracted.11 Secondly, Architect® has better metabolite cross-reactivity. This cross-reactivity is always positive with metabolites F2 (8.7%), F3 (4.1%), F4 (36.8%) and F5 (20.3%) (data provided by Abbott Laboratories®). For IMx®, these interferences are lower: F2 (2.8%), F4 (26.2%) and F5 (6.8%), but higher with F3, and, also, negative (-22%). This difference was extended when we directly compare IMx® and Architect®.
The decrease in QL from 2.5ng/ml (IMx®) to 0.7ng/ml (Architect®) allows for regimen adjustment when lower levels are required.1
Recently, the laboratory that markets sirolimus sent a communication to health care professionals warning of the changes made to immunoassays and the consequences that this has on monitoring levels.12 This communication especially emphasised the need for doctors to contact the laboratory to find out which assay is used, given that changes between different immunoassays or between one immunoassay and HPLC could produce clinically significant differences in results. These differences could provoke inadequate dosage adjustments, possibly causing adverse consequences. In our study, IMx® had more infra-therapeutic results than Architect® (52% vs. 19%), which could mean that there more patients’ doses would be increased than with Architect®.
To date, therapeutic windows have not been standardised for each measurement technique. Recently, the University of Colorado Hospital tried to adapt this therapeutic windows.13 Given that the levels obtained by Architect® are higher, the window has increased from 3-8ng/ml (with HPLC) to 4.5-13ng/ml (with Architect®).
Our study’s most significant limitation is that we have included a small amount of measurements in the sample, which could not have been increased as Abbott Laboratories® stopped marketing the IMx® reagent. Furthermore, our study includes the most kidney transplant patients to date.
It confirms that the laboratories that determine the sirolimus levels should inform doctors when they make changes to the immunoassay employed, and the consequences that could arise. This information is of vital importance so that appropriate dose adjustments can be made. Furthermore, this information should be considered when conducting clinical studies or comparisons between different hospitals. Similarly, sirolimus therapeutic windows should be standardised for each of the techniques in use.
Figure 1. Bland-Altman graph showing the sirolimus concentration differences between IMx® and Architect® (n=21 samples)
Figure 2. Linear correlation between sirolimus concentrations of IMx® and Architect® (n=21)