To the Editor,
In patients diagnosed with systemic lupus erythematosus (SLE) and nephrotic syndrome, the most common histological findings are diffuse proliferative glomerulonephritis and membranous nephropathy (class IV and class V according to the World Health Organisation [WHO], 1982).1
Here we report the case of a patient with SLE who developed nephrotic syndrome secondary to minimal-change nephropathy (MCN).
Our patient was a 59 year-old woman diagnosed with SLE (photosensitivity, discoid lupus, arthritis, and positive test for antinuclear antibodies [ANA]) 18 years earlier, occasionally under treatment with non-steroidal anti-inflammatory drugs (NSAID) and hydroxychloroquine. The patient was referred to our department for oedema in the legs and arterial hypertension for the past two months. The physical examination revealed oedema in both legs up to the knee and a blood pressure of 140/90mm Hg. The laboratory analysis revealed normal haemoglobin, leukocyte, and platelet values; creatinine: 0.75mg/dl; urea: 31mg/dl; total cholesterol: 241mg/dl; albumin: 3.2g/dl; ANA+: 1/640 (several years earlier: +1/320); anti-Ro: 127.9U/ml; normal/negative results for lupus anti-coagulants, anti-cardiolipin antibodies, anti-DNA, ENA, anti-La, and C3-C4; proteinogram without a monoclonal peak; proteinuria: 6.2g/24 hours (high-resolution electrophoresis [HRE]: selective glomerular pattern); urinary sediment: 12-20 red blood cells/field; negative urine culture. Serology for hepatitis B and C, HIV, and Epstein-Barr was negative; cytomegalovirus: previous exposure, negative RPR. Chest x-ray, echocardiogram, renal ultrasound, mammography, thoracic/abdominal/pelvic tomography, and gastroscopy were without relevant abnormalities.
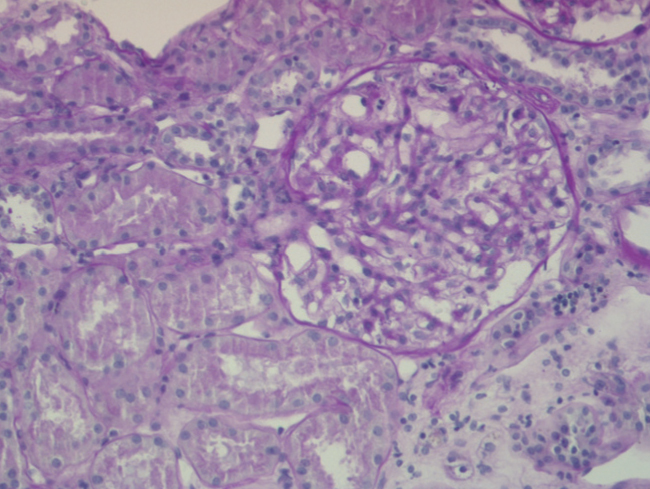
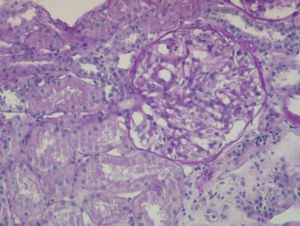
The percutaneous renal biopsy (13 glomeruli) showed 2/3 normal glomeruli and 1/3 with mesangial widening, with no thickening of the capillary walls or other lesions (Figure 1); the immunofluorescence analysis revealed IgM mesangial deposits in less than 1 third of the glomeruli, with no IgG or complement; the electron microscope analysis found diffuse effacement of pedicels and an absence of electron-dense deposits, all of which was compatible with MCN.
We administered prednisone 50mg/day for 8 weeks in a progressively decreasing pattern. Proteinuria decreased progressively, with partial/complete remission 14 months after the diagnosis (proteinuria: 480mg/24h).
MCN is uncommon in SLE, with a prevalence <2% in adults.2,3
MCN can appear in SLE along with the initial diagnosis or during a flare, but can also occur several years after the onset of SLE and without any apparent lupus activity.2 It is still under debate whether this is a causal relationship or some sort of histological sub-class of lupus nephritis. In principle, the appearance of two autoimmune diseases in the same patient suggests linked pathogenesis. On the other hand, some authors2,4 estimate that the prevalence of MCN in SLE is much higher than in the general population, and that SLE could be the causal factor. Thus, MCN could be a lupus podocytopathy associated with T-lymphocyte alterations in SLE.4 However, other authors suggest that idiopathic MCN can appear in patients with SLE that are in remission.5
In the current classification system for lupus nephritis (ISN/RPS, 2004), minimal mesangial lupus nephritis is established as class I, characterised by normal glomeruli in optical microscopy analysis and mesangial deposits in immunofluorescence tests and/or electron microscopy analysis.6 Cases of MCN in SLE have been described such as ours in which no significant deposits are observed in immunofluorescence or electron microscopy analyses,5,7 and that do not fit the current classification for lupus nephritis. However, according to the WHO classification system, they could be categorised as class Ia.
In this patient, we observed no temporal relationship with NSAID treatment.
To conclude, SLE appears to be a precipitating factor for MCN. However, in certain cases such as ours, in which MCN appears several years after SLE onset, with little or no lupus activity and no deposits observed in the renal biopsy samples, the pathogenic relationship is more debatable.
Acknowledgements
We would like to thank Professor Jerónimo Forteza, head of the histopathology department at the Hospital Clínico Universitario de Santiago de Compostela, for his invaluable assistance with the electron microscopy analysis.
Conflicts of interest
The authors affirm that they have no conflicts of interest related to the content of this article.
Figure 1. Percutaneous renal biopsy