There is enough evidence concerning the short-term safety of living donors after kidney transplantation. However, long-term complications continue to be studied, with a particular interest in young donors. Previous studies have been conducted in older donors for adult renal patients. We present a study of long-term complications in kidney donors for our paediatric population.
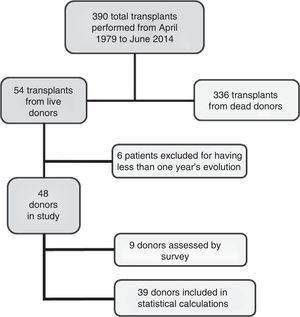
MethodsWe carried out a long-term donor study for the 54 living kidney-donor transplantations performed at our department from 1979 to June 2014. We monitored the glomerular filtration rate (GFR) on the basis of 24-h urine creatinine clearance, 24-h proteinuria and the development of arterial hypertension in the 48 donors who were followed up for more than one year. Only the 39 patients who were exclusively followed up by our department have been included in the results analysis.
ResultsGFR through creatinine clearance was stable after an initial decrease. No proteinuria was observed in any of the cases. One patient developed chronic kidney disease (CKD), which resulted in a cumulative incidence of 2%. GFR below 60mL/min/1.73m2 was not reported in any other patients. Arterial hypertension was diagnosed in 25% of donors, 90% of which were treated with antihypertensives.
ConclusionsRisk of CKD and hypertension in living kidney donors for paediatric recipients, who are carefully monitored throughout their evolution, is similar to that of the general population. Therefore, this technique appears to be safe in both the short and long term.
Existe evidencia suficiente sobre la seguridad a corto plazo en el donante vivo tras el trasplante renal. Sin embargo, las complicaciones a largo plazo continúan siendo un área de estudio en la actualidad, de especial interés en el donante joven. Previamente se han realizado análisis en donantes de edad más avanzada para enfermos renales adultos. Presentamos un estudio de complicaciones a largo plazo en donantes renales para nuestra población pediátrica.
MétodosEstudiamos a largo plazo a los donantes de los 54 trasplantes renales de donante vivo realizados en nuestro servicio desde 1979 hasta junio del 2014. Hemos monitorizado el filtrado glomerular (FG) mediante aclaramiento de creatinina en orina de 24h, proteinuria en orina de 24h y el desarrollo de hipertensión arterial (HTA) en los 48 donantes que han presentado un seguimiento mayor de un año. En el análisis de resultados se han incluido tan solo a los 39 pacientes que han sido exclusivamente controlados en nuestro servicio.
ResultadosEl FG mediante aclaramiento de creatinina fue estable tras su descenso inicial. No se observó proteinuria en ninguno de los casos. Se observó enfermedad renal crónica (ERC) avanzada en un paciente, lo cual supuso una incidencia acumulada del 2%, no describiéndose FG menor de 60mL/min/1,73m2 en ningún otro paciente. La HTA fue diagnosticada en el 25% de los donantes, con tratamiento hipotensor en el 90% de ellos.
ConclusionesEl riesgo de ERC y de HTA en donantes renales vivos para receptor pediátrico cuidadosamente monitorizados en su evolución es similar al de la población general por lo que parece tratarse de una técnica segura a corto y largo plazo.
Living donor kidney transplantation (LDKT) is the treatment of choice for children with advanced chronic kidney disease. It reduces the waiting time for transplant, the morbidity/mortality, and it improves the survival for both the graft and the recipient.1,2 The use of LDKT has increased in recent years for various reasons. There is an increased in the prevalence of advanced chronic kidney disease3 and there is decrease in the number of suitable brain-dead donors (a factor that especially affects children awaiting for a transplant),4 and in addition the procedure for donors5 has been improved in safety and short-term risks of the intervention have been minimised with the use of laparoscopic surgery. Nevertheless, since the main objective of LDKT is to ensure the safety of the donor, the scientific community has recently become interested in the possible long-term complications of donors.
Retrospective cohort studies with short follow-up suggest that a donors survival is similar to the general population, or even longer.6 Recently, however, long follow up studies have been published with populations that are especially healthy compared to the population of kidney donors, and these studies point to an increased incidence of chronic kidney disease and long-term mortality.7–13
Therefore the long-term risk of complications is an area of special interest for transplants in the paediatric population, because the donors are usually their parents which are usually young4 that should have a long survival. These donors may be exposed to the complications derived from having only one kidney.
The objective of this study is to assess the long-term consequences of nephrectomies in our donor population for LDKT in our Paediatric Nephrology Department. To this end, we have analysed the evolution of the patients (donors), glomerular filtration (GF), blood pressure (BP), incidence of hypertension (HT) and the existence of periodic proteinuria or microalbuminuria as markers of kidney pathology.
Material and methodsWe present a single-centre prospective cohort study. From the time our paediatric kidney transplant department opened in April 1979 to June 2014, our department has performed 390 transplants, 54 of them were from living donors. All donors were relatives: 30 mothers, 22 fathers, one sibling and one aunt.
Of the total group of 54 donors, 6 were excluded because they had a follow-up period of less than one year when the study closed in June 2014. Thus, the initial group included 48 donors with a minimum one-year follow-up period. The average age of the donors was 38 (18–51), and the average follow-up period was 12.5 years (1–30). Of these 48 donors, we evaluated the evolution and final status of 9 of them using a telephone and/or personal survey because they do not live in our area. We verified their evolution and clinical status at the end of the study using their clinical records and an assessment of their blood chemistries, to rule out kidney pathologies and to define kidney function. Nevertheless, we did not include these patients in the final statistical analysis because clinical examinations and tests were not conducted by ourselves. Therefore, our analysis focuses on the other 39 donors (Fig. 1).
Informed consent was obtained from all of donors, and the study was approved by our hospital's ethics committee.
Both the pre-nephrectomy study of the donors to assess their clinical status, and the subsequent follow-up study were performed in our Paediatric Nephrology Department. The preliminary study excluded donors presenting systemic, psychological or kidney pathologies that contraindicated donation, and this was confirmed by the Internal Medicine Department in accordance with the recommended guidelines for being kidney donors.14 Up to the present time, 40% of the possible donors have been ruled out for medical and/or psychological reasons due to the strict evaluation of requirements needed to qualify as donor.
A post-donation follow-up protocol is followed that includes periodic visits for medical interviews, blood tests to assess kidney and systemic pathologies such as dyslipidemia, diabetes and obesity that can negatively affect kidney evolution. We also monitor the donors’ BP, calculate their GFR l and investigate the presence of proteinuria. The frequency of these examinations is quarterly during the first year and annual or bi-annual thereafter, coinciding with the clinical visits of the recipients.
The patients’ BP was measured 3 consecutive times at rest in the hospital's outpatient clinic and by out patient monitoring. We define hypertension (HTN) as a systolic BP of 140mmHg or higher, a diastolic BP of 90mmHg or and/or treatment with anti-hypertensive drugs.6
The patients’ GFR are estimated by calculating the creatinine clearance rate (CCr) using their 24-h urine adjusted for their body surface area (mL/min/1.73m2). Proteinuria is tested in 24-h urine and it is defined as >300mg/day, with urinary proteinuria between 30 and 300mg/day classified as microalbuminuria.
The results are presented as median±standard deviation and/or range. The statistical significance of non-parameter data was analysed using a Student's t-test. The cumulative incidence is expressed as a percentage (Table 1).
Findings.
| LDKT donors (n=39) | |
|---|---|
| Average age (max-min) (years) | 38 (18–51) |
| Male/female (n) | 22/17 |
| Relatives (%) | 100 |
| Average follow-up period (min-max) (years) | 12.5 (1–30) |
| GF (CCr) (ml/min/1.73m2) | |
| Before 1 year -5 years -10 years -15 years | 128-101-100-98-110 |
| Serum Cr (mg/dL) | |
| Before 1 year -5 years -10 years -15 years | 0.86-1.06-1.03-1.03-1.07 |
| SBP/DBP (mmHg) | |
| Before 1 year -5 years -10 years -15 years | 111/70-116/73-118/74-126/80 |
| Microalbuminuria (μg/min) | |
| Before 1 year -5 years -10 years -15 years | 2.1-8.2-19.7-35.4-35.9 |
The sample set comprises 39 donors with full data collected in our health centre.
CCr, creatinine clearance; GF, glomerular filtration; n, sample; DBP, diastolic blood pressure; SBP, systolic blood pressure; LDKT, live donor kidney transplant.
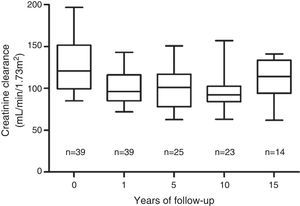
The most stable parameter over time was CCr. The mean pre-transplantation was 128mL/min/1.73m2±30mL/min/1.73m2. After an initial decrease from the pre-nephrectomy level, the GF level stabilised one year after the transplant at 100mL/min/1.73m2±20mL/min/1.73m2, and this value was maintained during 15 years of the follow-up period (Fig. 2). A CCr below 60mL/min/1.73m2 was not observed in any case. Proteinuria, defined as albuminuria >300mg/day, was not detected in any of the donors.
One case of advanced kidney disease was recorded after telephone interview and later on in person. This donor refused follow up and 18 years after the nephrectomy he was diagnosed with HTN, proteinuria and dyslipidaemia in an outpatient examination. He refused treatment and subsequent follow-up. The patient presented with progressive renal insufficiency with a need for dialysis 28 years after the nephrectomy, having received a kidney transplant at age 60 (33 years post-nephrectomy). The recipient (his son) currently has normal function of his transplant 33 years after the transplantation, with creatinine below 1mg/dl, with an underlying condition of renal dysplasia with malformed uropathy.
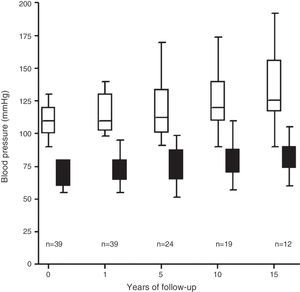
HypertensionThe average pre-donation systolic blood pressure was 118±19mmHg and the average diastolic blood pressure was 74±12mmHg. The donors’ BP increased over the follow-up period with an HT diagnosis criterion in 10 of the 39 donors studied (25%) (Fig. 3), 90% of whom were prescribed treatment with anti-hypertension medication.
DiscussionOur analysis revealed stability of GFR in our study population of 15 years of follow-up, after an immediate initial decrease attributed to loss of renal parenchyma, as normally observed in other series.15 We observed a progression to advanced renal disease in one of the 48 donors (2.01%.) The patient refused follow-up and treatment with a need for replacement kidney therapy 28 years post-nephrectomy. None of the other patients had a GFR below 60mL/min/1.73m2, with a prevalence of stage 3–4 CKD of less than the 3.3% described for the general population with similar characteristics.16
The development of advanced renal failure in one case – with no underlying conditions related to the recipient's TKD, and which is supposedly related to the harm induced by the patient's hypertension, dyslipidaemia and obesity that was noted in his clinical history – substantiates and confirms the need to closely monitor donors post-nephrectomy, as this may prevent the progression to terminal renal failure.
Numerous previous studies, mostly in the United States population, have evaluated renal function, describing terminal renal failure prevalences of 0.15–0.87%, and CKD prevalences of 5.2–14.5%.7,9,15,17–19 A large majority of these studies are based on retrospective cohorts taken from state transplant records, with short evolution periods and compared to the general population that is taken as a control group. Nevertheless, since the donor community is a particularly healthy group, recent findings have been added from studies that used control groups from a specially selected population that is more comparable with the health characteristics of a donor population.20–22 Mjoen et al. found a 0.31% cumulative incidence of CKD in a prospective cohort of 1901 Norwegian donors with a long follow-up period of 15 years and a hazard ratio of 11.4 vs. an control population that was particularly healthy. Likewise, Muzaale et al. observed a cumulative CKD incidence of 0.31% in live donors compared to 0.03% in a healthy control group.
The cumulative incidence of HT in our study was 25%: which is inferior to values in the general population with the same age range reported by Banegas in a cross-sectional study of the Spanish population.23 With active follow-up, all our patients were aware of their hypertension, and 90% of the hypertensive patients were treated with anti-hypertension medication. Studies of kidney donors also describe comparable HT prevalences among the general population.15,17
The transplant community has expressed its concern about the long-term complications that might arise from kidney transplants. In recent years, concern has been particularly focused on young donors.24,25 These donors have a longer exposure to a reduction of renal parenchyma, and to the complications this may entail. Moreover, since they have a high survival rate, they also have a higher risk of onset of medical pathologies that may lead to subsequent kidney disease. In this regard, in 2008 Gibney et al. described a higher risk of terminal renal failure in their donors under the age of 35.25 The average age of our paediatric donors is 38, so they are 13 years younger than the donors for adult patients.4 They are therefore a cohort that is of great interest for studying long-term kidney complications in this population.
Although our study does present a small number of donors compared to the series published for transplantations in adults, its long follow up and its prospective character make it especially interesting for assessing the risk of HT and/or the onset of CKD. As a single-centre study, both the pre- and post-nephrectomy studies were conducted in our Paediatric Nephrology Department, which made collecting and compiling the study's clinical and analytical data uniform and homogeneous. Thus, 9 of the donors whom we did not examine directly were ruled out, instead merely assessing their final status with regard to their clinical situation.
In conclusion, our findings show that the incidence of CKD, HT and renal function burden in donors may be similar to the general population, and that kidney donation for paediatric kidney disease patients is reasonably safe if it is associated with strict controls prior to the nephrectomy and thereafter that would make it possible to diagnose, control and early treatment for any possible complications. Long follow up studies will be necessary to evaluate a higher survival rate in paediatric vs. adult donors.
Conflicts of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: Martin J, Román E, Mendizabal S. Seguridad a largo plazo en el donante vivo para trasplante renal pediátrico. Estudio prospectivo, unicéntrico. Nefrología. 2016;36:674–678.