This post hoc study analysed the perception of the relevance of chronic kidney disease (CKD) in dyslipidaemia screening and the choice of statin among primary care physicians (PCPs) and other specialists through a Delphi questionnaire.
MethodsThe questionnaire included 4 blocks of questions concerning dyslipidaemic patients with impaired carbohydrate metabolism. This study presents the results of the impact of CKD on screening and the choice of statin.
ResultsOf the 497 experts included, 58% were PCPs and 42% were specialists (35, 7% were nephrologists). There was consensus by both PCPs and specialists, with no difference between PCPs and specialists, that CKD patients should undergo a dyslipidaemia screening and that the screening should be part of routine clinical practice. However, there was no consensus in considering the estimated glomerular filtration rate (eGFR) (although there was consensus among PCPs and nephrologists), or considering albuminuria when selecting a statin, or in determining albuminuria during follow-up after having initiated treatment with statins (although there was consensus among the nephrologists).
ConclusionsThe consensus to analyse the lipid profile in CKD patients suggests acknowledgement of the high cardiovascular risk of this condition. However, the lack of consensus in considering renal function or albuminuria, both when selecting a statin and during follow-up, suggests a limited knowledge of the differences between statins in relation to CKD. Thus, it would be advisable to develop a guideline/consensus document on the use of statins in CKD.
Este estudio post hoc analizó la percepción de la importancia de la enfermedad renal crónica (ERC) en el cribado de la dislipidemia y en la elección del tratamiento con estatinas entre médicos de Atención Primaria (MAP) y otras especialidades mediante cuestionario Delphi.
MétodosEl cuestionario incluyó 4bloques de preguntas alrededor del paciente dislipémico con alteración del metabolismo hidrocarbonado. Aquí se presentan los resultados relacionados con la consideración de la ERC en el cribado y la elección de la estatina.
ResultadosDe los 497 expertos incluidos, el 58% eran MAP y el 42%, especialistas (35, el 7%, nefrólogos). Hubo consenso en realizar un cribado de la dislipidemia en los pacientes con ERC, sin diferencias entre MAP y especialistas; y también en realizar el cribado en la práctica clínica habitual. Sin embargo, no se alcanzó el consenso en considerar el filtrado glomerular estimado (aunque sí entre MAP y nefrólogos) o la albuminuria en la elección de la estatina, ni en su determinación durante el seguimiento después de instaurar un tratamiento con estatinas (aunque hubo consenso entre nefrólogos).
ConclusionesEl consenso en analizar el perfil lipídico en los pacientes con ERC indica el reconocimiento del alto riesgo cardiovascular de esta enfermedad. La ausencia de acuerdo en considerar la función renal o la albuminuria, tanto en la elección de la estatina como durante el seguimiento, indica un conocimiento limitado de las diferencias entre estatinas en relación con la ERC, por lo que sería deseable disponer de una guía/documento de consenso sobre uso de estatinas en la ERC.
Patients with chronic kidney disease (CKD) have a high rate of cardiovascular morbidity and mortality1–3; therefore in different clinical guidelines this disease is considered a powerful and common predictor of cardiovascular events and mortality,4 which involves stratifying these patients as being at a high or very high risk of cardiovascular disease, and a tight control of different risk factors, including dyslipidaemia. Dyslipidaemia may contribute not only to the development of cardiovascular disease, but also to the onset and progression of CKD.5,6
HMG-CoA reductase inhibitors, or statins, are the most widely used lipid-lowering drugs for the management of dyslipidaemia. This is because the clear clinical evidence of their cardiovascular benefits, even in patients with CKD.7–9 In a recent systematic review and meta-analysis on the effects of statins in CKD patients, including 31 clinical trials with 48.429 patients, statin therapy achieved a 23% reduction in the risk of severe cardiovascular events, a reduction of 18% in the risk of coronary events and a 9% decreased risk of cardiovascular or total mortality.10 In addition, statins may delay the progression of CKD by reducing proteinuria, inflammation and fibrosis,11 although the evidence on this beneficial effect is limited and contradictory.10,12,13 However, one recent cohort study points to an increased risk of renal events with the use of statins.14 One recent consensus document by a panel of lipid experts on intolerance to statins recognises that statins may be associated with an increased risk of acute kidney failure and proteinuria.15
Not all statins are cleared uniformly by kidneys; some have a higher rate of renal excretion and require dose adjustments in CKD (e.g., pravastatin or rosuvastatin), while others have a significant risk of drug-related interactions16 this is a relevant fact for renal patients, who are usually polymedicated. Also, some evidence indicates that different statins may have different effects on urinary albumin excretion and the progression of kidney disease.17–21
The DIANA study was recently carried out to assess the knowledge and attitudes in relation to the diabetogenicity of the different statins, such as the differences that may exist amongst them, with a view to a more rational use in patients with type 2 diabetes mellitus or those predisposed to developing it.22 To determine the opinion of prescribing doctors about the screening and management of dyslipidaemia in patients with CKD, we performed a post hoc analysis of the DIANA study.
MethodsStudy designThe modified Delphi method23 was used to achieve the widest possible consensus of a broad panel of doctors experts in the management of dyslipidaemia. This is a structured technique of professional consensus which is achieved remotely, i.e., a variant of the original procedure developed by Dalkey et al.,24,25 which maintains its main advantages (controlled interaction amongst panel members, chance to reflect and reconsider one's opinion and statistical validation of the consensus achieved) compared with other technical alternatives, and which resolves some of its main drawbacks (biased opinions).26
The study required 2 successive rounds of a structured survey completed via an on-line platform. The expert doctors were able to confidentially contrast their personal opinions with the panel's aggregate opinion when responding to the second round and to reconsider, if deemed appropriate, their initial criteria on issues that had not been agreed upon.
The study was carried out in 4 phases: (a) formation of the scientific committee, responsible for proposing the panel of experts and creating the survey items; (b) formation of an expert panel of professionals from 5 medical specialties (cardiology, endocrinology, internal medicine, nephrology, and family and community medicine), with a special interest and experience in the field of dyslipidaemia, with the exclusive task of completing the survey; (c) on-line survey in 2 rounds; and (d) compilation, analysis of results and discussion of conclusions in a face-to-face session of the scientific committee.
Development of the questionnaireThe authors of this study made up the project's scientific committee owing to their individual career and professional experience in this field. Along with the collaboration of an external consultant on methodology, they developed the content of the Delphi questionnaire. For this, a bibliographic search was carried out, in which systematic meta-analyses/reviews and another type of critical synthesis of the scientific literature were prioritised through the use of standard bibliographic databases27 (MEDLINE, EMBASE and the Spanish Medical Index), as well as a manual review of the bibliographical references obtained to identify others that may be of interest based on keywords such as dyslipidaemia, diabetes mellitus or impaired glucose metabolism.
Each item of the survey evaluated by the panel was drafted by taking into account that it was an assertion, affirmative or negative, as a professional criterion or clinical recommendation, which would respond to aspects of interest or controversy in the clinical management of patients with dyslipidaemia and impaired glucose metabolism. The final version of the questionnaire included 4 blocks of questions:
- 1.
Algorithm for current management of dyslipidaemia, in particular in patients with impaired glucose metabolism: detection, therapeutic approach, monitoring and follow-up (57 items).
- 2.
Opinion on the relative importance of the factors taken into account when prescribing and following up on statin therapy (39 items).
- 3.
Opinion on the profile of statins in the treatment of dyslipidaemia in patients with impaired glucose metabolism (16 items).
- 4.
Recommendations for selecting the hypolipidaemic treatment of choice for patients with impaired glucose metabolism (24 items).
A single type of rating scale was proposed for all questions: 9-point Likert-type ordinal (1: no agreement/never/not important/unnecessary/inappropriate; 9: complete agreement/always/very important/absolutely necessary/absolutely appropriate), according to the format developed in the RAND/UCLA Appropriateness Method User's Manual.27 The response categories were described by linguistic qualifiers in 3 areas (1–3: disagreement, never/almost never, not important, unnecessary, not/almost never appropriate; 4–6: neutral; 7–9: agreement, almost always/always, important, necessary, very/absolutely appropriate).
All questions had to be answered in order to obtain the opinion of all panellists participating in both rounds on all issues raised. However, the second round considered only those items in which no consensus was obtained in the previous round, that is, those questions that did not obtain at least 80% of responses grouped in scores 1–3 (consensus in disagreement) or 7–9 (consensus in agreement).
In this study we collected the results of the questions related to the screening and management of dyslipidaemia in patients with CKD.
Expert panel selectionPanel experts were proposed by the scientific committee with the criterion of being representatives of their medical specialty with decision-making on the clinical status of the study, professional recognition for their experience and scientific opinion (leadership in the subject-matter) and special interest in the field of dyslipidaemia. For their identification, a “snowball” strategy was used based on the personal contacts of the committee's members, who in turn proposed new relevant candidates in their professional settings.28 Following this process, 506 professionals were invited. Of those 497 experts agreed to take part from across the Spanish autonomous communities. The study was carried out between February and June 2015, with electronic mail as a means of distribution.
Before beginning to answer the questionnaire, the experts had to answer a series of questions about their medical specialty, years of professional practice, and number and characteristics of patients with dyslipidaemia treated, amongst others.
Analysis and interpretation of the resultsIn order to analyse the group opinion regarding each question raised and for the interpretative purposes of the Likert-type scale questions, the presentation of the answers was systematised by grouping the range of possible values between 1 and 9 into 3 levels29: 1–3, 4–6, 7–9 (Fig. 1). A consensus was defined as one reached in disagreement or agreement when at least 80% of the panellists had given scores of 1 to 3 (consensus in disagreement) or 7 to 9 (agreement in agreement), respectively.
The data were analysed as a whole and according to the specialty of the participating doctors, comparing the answers of general practitioners (GPs) with those of specialist doctors. The comparative analysis by specialty was performed using the χ2 or Fisher's exact tests. To perform the comparative analysis between both rounds, Bowker's test30 was used, adapting McNemar's test to compare endpoints of more than 2 categories. In both cases, the level of statistical significance was 0.05 (two-tailed). The data were analysed using the statistical package SAS v.9.2 (SAS Institute Inc., Cary, NC, USA).
ResultsOf the 497 experts participating in the study, 58% were GPs and 42% specialist doctors (14% endocrinologists, 14% internists, 7% cardiologists and 7% nephrologists). Of the total number of experts participating, 62% had more than 20 years of professional practice and 80% cared on average for more than 50 dyslipidaemic patients a month. In addition, more than 60% of the participants reported that more than 50% of the patients treated in their offices were over 65 years of age, were polymedicated or had hypertension.
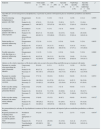
Table 1 shows the results obtained. There was a consensus (agreement of >80% of panellists) on the need for screening for dyslipidaemia in patients with CKD in the entire group (89.7%), with no statistically significant difference between GPs and specialists (87.9% vs. 92.4%, respectively; p=NS), nor any differences between nephrologists (91.4%) and non-nephrologists (92.4%; p=NS).
Level of agreement reached after 2 rounds by the participating experts.
| Endpoint | Response | Total sample (n=497) n (%) | GPs (n=290) n (%) | Specialty (n=207) n (%) | Nephrology specialty (n=35) n (%) | Specialty other than nephrology (n=172) n (%) | p |
|---|---|---|---|---|---|---|---|
| Algorithm for current management of dyslipidaemia, in particular in patients with impaired glucose metabolism (prediabetes and diabetes mellitus) | |||||||
| Need for detecting dyslipidaemia in patients with CKD | Disagreement (1–3) | 6 (1.2) | 3 (1.0) | 3 (1.4) | 1 (2.9) | 2 (1.2) | 0.3053 |
| Neutral (4–6) | 45 (9.1) | 32 (11.0) | 13 (6.3) | 2 (5.7) | 11 (6.4) | ||
| Agreement (7–9) | 446 (89.7) | 255 (87.9) | 191 (92.3) | 32 (91.4) | 159 (92.4) | ||
| Screening for dyslipidaemia in patients with CKD in clinical practice | Never/almost never (1–3) | 7 (1.4) | 4 (1.4) | 3 (1.4) | 0 (0.0) | 3 (1.7) | 0.6029 |
| Neutral (4–6) | 86 (17.3) | 54 (18.6) | 32 (15.5) | 3 (8.6) | 29 (16.9) | ||
| Almost always/always (7–9) | 404 (81.3) | 232 (80.0) | 172 (83.1) | 32 (91.4) | 140 (81.4) | ||
| Patient profile as a parameter influencing choice of statin | Disagreement (1–3) | 12 (2.4) | 4 (1.4) | 8 (3.9) | 3 (8.6) | 5 (2.9) | 0.0635 |
| Neutral (4–6) | 53 (10.7) | 34 (11.7) | 19 (9.2) | 5 (14.3) | 14 (8.1) | ||
| Agreement (7–9) | 432 (86.9) | 252 (86.9) | 180 (87.0) | 27 (77.1) | 153 (89.0) | ||
| Possible interaction with other drugs as a parameter influencing choice of statin | Disagreement (1–3) | 11 (2.2) | 3 (1.0) | 8 (3.9) | 3 (8.6) | 5 (2.9) | 0.0882 |
| Neutral (4–6) | 64 (12.9) | 38 (13.1) | 26 (12.6) | 3 (8.6) | 23 (13.4) | ||
| Agreement (7–9) | 422 (84.9) | 249 (85.9) | 173 (83.6) | 29 (82.9) | 144 (83.7) | ||
| Opinion on the relative importance of the factors taken into account when prescribing and following up on treatment with statins | |||||||
| Parameter to consider when prescribing the statin: glomerular filtration | Unnecessary (1–3) | 20 (4.0) | 9 (3.1) | 11 (5.3) | 1 (2.9) | 10 (5.8) | 0.0458b |
| Neutral (4–6) | 86 (17.3) | 42 (14.5) | 44 (21.3) | 4 (11.4) | 40 (23.3) | ||
| Necessary (7–9) | 391 (78.7) | 239 (82.4) | 152 (73.4) | 30 (85.7) | 122 (70.9) | ||
| Parameter to consider when prescribing the statin: urinary albumin excretion | Unnecessary (1–3) | 37 (7.4) | 18 (6.2) | 19 (9.2) | 0 (0.0) | 19 (11.0) | 0.0513 |
| Neutral (4–6) | 146 (29.4) | 81 (27.9) | 65 (31.4) | 9 (25.7) | 56 (32.6) | ||
| Necessary (7–9) | 314 (63.2) | 191 (65.9) | 123 (59.4) | 26 (74.3) | 97 (56.4) | ||
| Follow-up after starting treatment with statins: glomerular filtration | Unnecessary (1–3) | 15 (3.0) | 12 (4.1) | 3 (1.4) | 0 (0.0) | 3 (1.7) | 0.1704 |
| Neutral (4–6) | 94 (18.9) | 51 (17.6) | 43 (20.8) | 4 (11.4) | 39 (22.7) | ||
| Necessary (7–9) | 388 (78.1) | 227 (78.3) | 161 (77.8) | 31 (88.6) | 130 (75.6) | ||
| Follow-up after starting treatment with statins: urinary albumin excretion | Unnecessary (1–3) | 33 (6.6) | 15 (5.2) | 18 (8.7) | 0 (0.0) | 18 (10.5) | 0.0112b |
| Neutral (4–6) | 140 (28.2) | 79 (27.2) | 61 (29.5) | 6 (17.1) | 55 (32.0) | ||
| Necessary (7–9) | 324 (65.2) | 196 (67.6) | 128 (61.8) | 29 (82.9) | 99 (57.6) | ||
| Recommendations for selecting the hypolipidaemic treatment of choice for patients with impaired glucose metabolism (prediabetes or diabetes mellitus) | |||||||
| Required parameters for evaluating when deciding: Take into account the patient's renal function/albuminuria | Not/almost never appropriate (1–3) | 10 (2.0) | 3 (1.0) | 7 (3.4) | 2 (5.7) | 5 (2.9) | 0.0314b |
| Neutral (4–6) | 57 (11.5) | 26 (9.0) | 31 (15.0) | 4 (11.4) | 27 (15.7) | ||
| Very/absolutely appropriate (7–9) | 430 (86.5) | 261 (90.0)a | 169 (81.6) | 29 (82.9) | 140 (81.4) | ||
CKD: chronic kidney disease; GPs: general practitioners.
Agreement was also reached on when to screen for dyslipidaemia in patients with CKD in clinical practice, because 81.3% of the doctors surveyed stated that they performed this always/almost always, with no statistically significant differences between GPs and specialists (80.0% vs. 83.1%, respectively; p=NS). Although the differences were not significant, nephrologists responded more often that they screened always/almost always (91.4%) than non-nephrologist specialists (81.4%).
Regarding which parameters influence the choice of statin, there was agreement in which, amongst other aspects, patient profile influences the choice of statin (86.9%), as well as possible interaction with other drugs (84.9%), with no statistically significant differences between GPs and specialists (85.9 vs. 83.6%). When the data were analysed by differentiating between nephrologists and non-nephrologists, the former showed no consensus (77.1%), whereas the latter did show consensus (89.0%), with a similar figure for GPs (86.9%; p=0.0635). There was no difference between subgroups, and there was agreement amongst all of them when considering the patients’ polymedicated status on choosing a statin.
However, regarding the parameters to be considered when deciding on a statin, there was no consensus in the entire sample when considering estimated glomerular filtration rate (eGFR) (78.7%; GPs 82.4% vs. specialists 73.4%; p=NS). When analysing this parameter by considering a given expert's specialty, as with the GPs, the nephrologists deemed it necessary to consider eGFR when deciding on a statin (85.7%), but the non-nephrologists did not (70.9%; p=0.0458). Nor was a consensus reached with regard to albuminuria in the entire sample analysed (63.2%), though the nephrologists seemed to take it into account more often (74.3% nephrologists vs. 56.4% non-nephrologists vs. 65.9% GPs; p=0.0513).
Also, no agreement was reached on measuring eGFR (78.1%: GPs 78.3% vs. specialists 77.8%; p=NS) or urinary albumin excretion (65.2%: GPs 67.6% vs. specialists 61.8%; p=NS) during follow-up after starting statin therapy. When the specialty was subdivided between nephrologists and non-nephrologists, the nephrologists considered evaluating eGFR (88.6%), whereas the non-nephrologists, as well as the GPs, did not reach a consensus (75.6%; p=0.1704). Something similar happened with albuminuria, but in this parameter there were statistically significant differences amongst the groups of experts (GPs 67.6%; 82.9% vs. nephrologists vs. 57.6% non-nephrologists; p=0.0112).
Regarding which parameters should be considered when prescribing a statin, it was considered that a patient's renal function/albuminuria should be accounted for (85.7%: GPs 90.0% vs. specialists 81.6%; p=0.0175), with no differences between specialist types (nephrologists 82.9 vs. 81.4% non-nephrologists).
DiscussionIn this post hoc analysis of the DIANA study, there was agreement that dyslipidaemia should be studied in patients with CKD and that it is routine practice to screen for it in this population. This means that the participating doctors recognised the high cardiovascular risk associated with CKD, as indicated in the guidelines and consensus documents.4,31 The Kidney Disease | Improving Global Outcomes (KDIGO) guide on managing dyslipidaemia in CKD recommends measuring the lipid profile in this specific population to establish a diagnosis of dyslipidaemia,32 which amongst nephrologists is an almost-universal practice. In addition, the study confirmed that renal function/albuminuria should be considered amongst the parameters for the choice of statin, as it would classify the patient as having a high or very high cardiovascular risk.
There was also agreement that patient profile, as well as possible interaction with other drugs, should be considered when choosing a statin. However, the nephrologists did not reach a consensus on whether patient profile influenced the choice of statin (77.1%). This may be due to the fact that, in general, renal patients seen by nephrologists are in more advanced stages of the disease, and these patients have a very high cardiovascular risk. Therefore, statin therapy is almost mandatory.32 In a word, prescribing doctors are aware that, in addition to considering the efficacy of a statin in meeting an LDL-C goal, they should consider other aspects related to patients and polymedication.
However, there was no consensus in considering eGFR or urinary albumin excretion as factors to be taken into account in the choice of statin. However, according to the subgroups of doctors surveyed, both GPs and nephrologists did reach a consensus in considering eGFR as an element to account for in the choice of statin, but this was not achieved in non-nephrologist specialists (70.9%), with a significant difference. This discrepancy may be due to different drug-prescription patterns amongst different specialists. It seems then that whereas GPs integrate the different prescribed treatments and clinical conditions and perform long-term patient monitoring, specialists often follow a fire-and-forget strategy, which is advocated by the latest US guidelines, which recommend starting statin therapy with fixed doses with no LDL-C goal.33 The KDIGO guidelines32 also recommend the use of a statin or statin/ezetimibe in patients with CKD who are older than 50 years with an eGFR<60ml/min/1.73m2. By contrast, the 2012 European guidelines on cardiovascular disease prevention in clinical practice34 consider patients with stage 4–5 CKD (eGFR<30ml/min/1.73m2) to have a very high cardiovascular risk, and they establish the same goal for monitoring such patients (LDL-C below 70mg/dl, or at least a 50% reduction compared with the baseline) as for patients with a history of atherosclerotic vascular disease (secondary prevention). Further, patients with stage 3 CKD (eGFR between 30 and 60ml/min/1.73m2) are classified as having a high cardiovascular risk and they are assigned an LDL-C goal of <100mg/dl. Alternatively, it could be inferred that amongst non-nephrologist specialists there is less knowledge that some statins show greater renal clearance and therefore require dose adjustments.
It is also surprising that there is no consensus in considering baseline albuminuria amongst any of the groups of doctors, although there are studies that indicate that statins are not homogeneous in this regard.20,21
All of this indicates the need for a better understanding of the implications of CKD in prescribing statins amongst specialists in order to reduce cardiovascular risk and minimise the risk of renal adverse effects in this population.15
Nor was it considered necessary to monitor renal function or urinary albumin excretion during follow-up after prescribing a statin, nor amongst GPs or specialists, although when analysed by subgroup, the nephrologists reached a consensus in evaluating eGFR and albuminuria during follow-up of their patients. In this regard, some observational studies have shown an increased risk of renal events (acute kidney failure) with the use of statins,14,35,36 but this has not been observed in other clinical trials, prospective studies or meta-analyses.10,12,13,37
Although it may seem that the effects of statins on renal function are minor, many of the positive studies stem from the analysis of clinical trials designed to evaluate cardiovascular events in which the measure of renal function was limited to the eGFR. With respect to specific statins, some evidence indicates a beneficial effect on renal function in some,17,19 and a neutral effect in others.12 In comparative clinical trials of statins, differences have been observed amongst them in terms of progression of CKD21 or of reduction of albuminuria.20 This seems to be known in particular amongst nephrologists, and to a lesser extent amongst GPs and non-nephrologists. Finally, a recent meta-analysis indicates that high-efficacy statins might have a beneficial effect on decreased renal function (but not those of medium-low efficacy) in patients with CKD.38
As mentioned above, patients with CKD have a higher risk of developing adverse effects when treated with statins15; therefore it is important to choose a statin with sufficient hypocholesterolaemic efficacy, with no risk of accumulation in the presence of CKD, and with a low risk of drug interactions that, if possible, have shown a beneficial effect on the progression of CKD. In this regard the European guidelines for the treatment of dyslipidaemia recommend the use of statins that show a lower renal excretion, such as fluvastatin, atorvastatin and pitavastatin, in patients with CKD,39 although they do not refer to dose adjustments in the presence of CKD. The Spanish consensus document on CKD is similar in this sense.31 Also in this vein, the KDIGO guidelines include dose adjustments for each statin in patients with CKD.32 It should also be noted that statins metabolised by cytochrome P450 3A4 (lovastatin, simvastatin and atorvastatin) may cause adverse reactions due to their interaction with other drugs commonly used for these patients. The lack of consensus in the recommendations on the type and maximum dose of statin in CKD, especially in advanced CKD, and on the relationship between statins and proteinuria/albuminuria is therefore evident. Therefore a consensus document/guide to advise specialists in the choice of statin in CKD would be useful.
This study's main limitation is that the questionnaire was not specifically designed to show whether the presence of CKD had any effect on screening for dyslipidaemia or on selecting a hypolipidaemic treatment. Nor did it specifically ask whether there are differences between statins regarding renal function or albuminuria, or regarding possible differences between high- and low-medium-efficacy statins; this should be addressed in a future study. However, the answers contained in this analysis are novel, based on clinical practice, and allow us to detect training-related deficiencies of prescribing doctors in terms of the appropriate choice of a statin in patients with CKD. By contrast, the number of nephrologists included is small, and therefore the results of the study may not be extrapolated to all nephrologists. The statistical power of the study is limited when analysing the data by nephrologist and non-nephrologist: a “false negative” may be obtained, i.e., maintaining that there are no differences amongst groups, when in fact differences exist.
In summary, the data from this study show that there is a consensus on the need to detect dyslipidaemia in CKD, thus indicating that doctors recognise that the condition involves stratifying patients as being at a high or very high cardiovascular risk, and therefore, the need to prescribe statins in this population. However, there does not appear to be adequate knowledge about the clinically relevant differences that exist amongst different statins in relation to CKD. Improving this situation, by developing a guide/consensus document and conducting training on the subject for different specialties, might allow doctors to choose the most suitable statin for each patient and to minimise the risk of adverse effects in this population.
FundingThis study was funded by Laboratorios Esteve, which did not, however, participate in the preparation of the survey, statistical analysis, discussion of results or the writing of the article, which were the responsibility of the committee of experts who signed as authors of the article.
Authorship/collaborationsAll the authors actively contributed to developing the study and drafting the manuscript, approving the final version and its submission for publication in Nefrología.
Conflicts of interestAll authors state that there are no conflicts of interest in the writing of the manuscript, and any type of financial or personal relationship that might interfere with the study has been specified.
We would like to thank the panellists surveyed as experts in the Delphi survey and Adelphi España (Barcelona), which was the company in charge of implementing the project, by assisting the scientific committee in the tasks of project design, statistical analysis and collection of final results.
Please cite this article as: Cases Amenós A, Pedro-Botet Montoya J, Pascual Fuster V, Barrios Alonso V, Pintó Sala X, Ascaso Gimilio JF, et al. Consenso Delphi sobre el diagnóstico y manejo de la dislipidemia en pacientes con enfermedad renal crónica: análisis post-hoc del estudio DIANA. Nefrologia. 2016;36:679–686.