Patients with chronic kidney disease (CKD) have an increased risk of adverse cardiovascular outcomes after non-ST elevation acute coronary syndrome (NSTE-ACS). However, the information available on this specific population is scarce. We evaluate the impact of CKD on long-term prognosis in patients with NSTE-ACS managed with invasive strategy.
MethodsWe conduct a prospective registry of patients with NSTE-ACS and coronary angiography. CKD was defined as a glomerular filtration rate <60ml/min/1.73m2. The composite primary end-point was cardiac death and non-fatal cardiovascular readmission. We estimated the cumulative probability and hazard rate (HR) of combined primary end-point at 3 years according to the presence or absence of CKD.
ResultsWe included 248 patients with mean age of 66.9 years, 25% women. CKD was present at baseline in 67 patients (27%). Patients with CKD were older (74.9 vs. 63.9 years; p<0.0001) with more prevalence of hypertension (89.6 vs. 66.3%; p<0.0001), diabetes (53.7 vs. 35.9%; p=0.011), history of heart failure (13.4 vs. 3.9%; p=0.006) and anemia (47.8 vs. 16%; p<0.0001). No differences in the extent of coronary artery disease. CKD was associated with higher cumulative probability (49.3 vs. 28.2%; log-rank p=0.001) and HR of the primary combined end-point (HR: 1.94; 95% CI: 1.12–3.27; p=0.012). CKD was an independent predictor of adverse cardiovascular outcomes at 3 years (HR: 1.66; 95% CI: 1.05–2.61; p=0.03).
ConclusionsIn NSTE-ACS patients treated with invasive strategy, CKD is associated independently with an increased risk of adverse cardiovascular outcomes at 3 years.
Los pacientes con enfermedad renal crónica (ERC) presentan mayor riesgo de eventos adversos cardiovasculares tras un síndrome coronario agudo sin elevación del segmento ST (SCASEST). Sin embargo, la información disponible en esta población específica es escasa. Evaluamos el efecto de la ERC en el pronóstico a largo plazo de pacientes con SCASEST tratados con estrategia invasiva.
MétodosRegistro prospectivo de pacientes con SCASEST y coronariografía. Definimos ERC como una tasa de filtrado glomerular < 60ml/min/1,73m2. La variable de valoración final fue el combinado de muerte y reingreso cardiovasculares (nuevo síndrome coronario agudo, insuficiencia cardíaca e ictus no fatales). Estimamos la probabilidad acumulada, estratificada por ERC, y la relación entre esta y la tasa de riesgo del evento combinado a 3 años.
ResultadosIncluimos a 248 pacientes, con media de edad de 66,9 años; el 25% eran mujeres. Los 67 casos (27%) con ERC fueron mayores (74,9 vs. 63,9 años; p<0,0001) y con más prevalencia de hipertensión (89,6 vs. 66,3%; p<0,0001), diabetes (53,7 vs. 35,9%; p=0,01), historia de insuficiencia cardíaca (13,4 vs. 3,9%; p=0,006) y anemia (47,8 vs. 16%; p<0,0001). Sin diferencias en la extensión de la enfermedad coronaria. La ERC se asoció a mayor probabilidad (49,3 vs. 28,2%; log-rank p=0,001) y tasa de riesgo del evento combinado (HR ajustada: 1,94; IC 95%: 1,12-3,27; p=0,012). La ERC fue predictor independiente de eventos (HR: 1,66; IC 95%: 1,05-2,61; p=0,03).
ConclusionesEn pacientes con SCASEST tratados con estrategia invasiva, la ERC se asocia de manera independiente a mayor riesgo de eventos cardiovasculares a 3 años.
Chronic kidney disease (CKD) includes a heterogeneous group of disorders that affect the structure and function of the kidney.1 Its prevalence continues to increase, reaching between 2.5% and 11.2% of the adult population in developed countries.2,3 In large registries of patients with acute coronary syndrome without ST segment elevation (NSTE-ACS) CKD is present in up to 40% of patients4; this is related to an increased risk of adverse cardiovascular events (ACVE), usually attributed to the higher frequency of traditional risk factors.2,5
In high-risk NSTE-ACS, results from randomized clinical trial suggest to perform an invasive strategy, with angiography and revascularization rather than being conservative (invasive procedures should be used if ischemia is demonstrated).6–9 The current guidelines recommend that the care offered to patients with CKD should not be affected by renal dysfunction unless drug dose adjustment is required.9 However, data from recent observational studies and registries show that in CKD cases with NSTE-ACS the diagnostic procedures that could benefit the patient are not always used.4 It would be debatable if differences in patient management could explain less favorable outcomes in CKD. The changes in prognosis as a consequence of applying an invasive strategy in patients with CKD are not uniform for all CKD stages.10,11 In addition, there are other metabolic factors that may favor endothelial dysfunction which directly contribute to poor prognosis.1,12
Many of the studies that evaluate patients with CKD in a NSTE-ACS are secondary analysis of clinical trials in which patients with moderate and advanced degrees of renal failure are excluded and these are patients usually seen in daily clinical practice not well represented in such studies.6,7,13 Others studies analyze the full spectrum of acute coronary syndrome, including or fully dedicated to dialysis patients, or only assess events during hospitalization or after short-term follow-up.14–17 All this implies that, in general the results of the published studies are of limited utility in populations of non-selected patients. The increase in the number of cases with NSTE-ACS that show CKD at admission and the scarce information in this population makes this subject particularly interesting.18
The present study evaluates the long-term effect of baseline renal function on the risk of presenting ACVE (adverse cardiovascular events) in a cohort of patients with NSTE-ACS using invasive therapeutic strategy. We focus on these patients, a homogenous population, to assess the specific influence of CKD on the results.
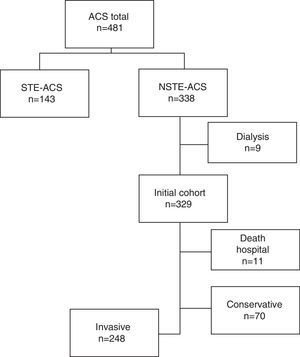
MethodsStudy populationData were collected from an observational clinical registry obtained under conditions of usual clinical practice in consecutive, non-selected patients with a diagnosis of acute coronary syndrome (ACS). We collected prospective data related to clinical characteristics, including physiological variables at admission, management strategies, procedures performed and events during follow-up. The initial cohort consisted of all patients older than 18 years admitted between November 1, 2011 and December 31, 2012 (n=481). We chose those patients with a diagnosis of NSTE-ACS (n=338), including unstable angina and myocardial infarction without ST segment elevation, considering the values of troponin I in admission. Patients who died in the hospital during the index episode (n=11) and those on regular dialysis prior to admission (n=9) were excluded. Of the remaining patients, we selected those in which the cardiologist decided to use an invasive strategy, defined by coronary angiography, with the intention of revascularization, during admission (N=248) (Fig. 1).
Measurements and definitionsThe glomerular filtration rate (eGFR) was estimated from the value of serum creatinine using equation of CKD-EPI.19 Baseline eGFR was calculated using the first available serum creatinine value measured in the hospital, that always was within the first 6h, and the eGFR at discharge was calculated using the last determination of serum creatinine obtained within the previous 48h before discharge. CKD20 was considered in those patients with eGFR≪60ml/min/1.73m2. Acute renal failure was defined according to the AKIN21 criteria based on creatinine changes between baseline and peak values and we considered stable renal function as a change in creatinine <0.3mg/dl from baseline.
Anemia was defined as the presence of baseline hemoglobin levels below 12g/dl in women and 13g/dl in men. Major bleeding was defined according to 3 of the criteria proposed by the Bleeding Academic Research Consortium, which, in summary, considers absolute hemoglobin drop ≥3g/dl from baseline, intracranial hemorrhage or any red blood cell transfusion.22 Significant coronary disease was considered in cases of estenosis >70% in at least one coronary artery. Optimal medical treatment (BMT) at discharge was the prescription of statins, angiotensin converting enzyme inhibitors/angiotensin II receptor antagonists, beta-blockers and antiplatelet agents. For the diagnosis of ACVE at follow-up, we use the definitions included in the recent given recommendations.23 For the analysis, it was considered the first event to happen.
The ACVE were assessed through electronic medical records, review of medical visits, hospital records, and telephone call if necessary. All patients had signed informed consent. The study protocol was approved by the Hospital's Ethics and Scientific Research Committee (ESRC) and it was performed in accordance with the ethical requirements expressed in the Helsinki Declaration and subsequent amendments.
The primary endpoint was to determine in CKD patients with NSTE-ACS treated invasively the risk of developing cardiovascular death and/or hospital admission due to new acute coronary syndrome, non-fatal heart failure and stroke during long-term follow-up (3 years) according to baseline eGFR.
Statistical analysisContinuous variables normally distributed were expressed as mean (SD) and median, and interquartile range (25–75) was used if the distribution was not normal. Percentage values were used for categorical variables and proportions. Patients were stratified according to the eGFR in: ≥60ml/min/1.73m2 and <60ml/min/1.73m2 and also in varying degrees of severity of CKD: ≥90ml/min/1.73m2 (normal), 60–89ml/min/1.73m2 (ERC), 45–59ml/min/1.73m2 (mild-moderate ERC) and <45ml/min/1.73m2 (moderate-serious). The continuous variables of the patients in each category were compared using Student's t test; the categorical variables were compared by χ2 test. The incidence rate of ACVE at 3-year follow-up after hospital discharge was estimated using the Kaplan–Meier survival curves and comparison between the group with and without CKD with the log rank test (Mantel-Cox). The relationship between degrees of GFR and the combined endpoint at follow-up was assessed using the Cox proportional hazards model adjusted for the effect of confounding factors. It was estimated whether the use of revascularization modified the relationship between CKD and ACVE during follow-up. Risk rates are expressed using the 95% confidence interval. To identify the independent predictors of ACVE, potential predictors’ variables with a p<0.10 in the univariant analysis were included in the analysis (hypertension, diabetes, history of heart failure and ischemic heart disease, dyslipidemia, anemia, major bleeding, CKD on admission) also included were: age, gender, revascularization during admission and TMO at discharge, which were incorporated with a stepwise selection procedure into a multivariable Cox regression model. Values of p<0.05 were considered statistically significant. The analyses were performed using IBM SPSS Statistics v.19.
ResultsThe baseline characteristics of the patients are summarized in Table 1. The mean age was 66.9 years and 25% were women. The prevalence of CKD at admission was 27% (n=67). The median GFR in this subgroup of patients was 47.7ml/min/1.73m2; 40.3% had moderate to severe renal dysfunction. Acute deterioration of renal function was renal failure diagnosed in 25 patients (10.1%), the majority (64%) in the CKD group (p<0.0001). The incidence of major bleeding was 4.8% and it was more frequent, but without statistical significance, among cases with renal failure (9 vs. 3.3%; p=0.07). Patients with baseline CKD were older and with higher prevalence of hypertension, diabetes, anemia and history of heart failure. In addition, the frequency of microalbuminuria and the degree of proteinuria were significantly increased.
Baseline characteristics, complications and treatment at discharge of the total population and stratified by eGFR.
| eGFR (ml/min/1.73m2) | ||||
|---|---|---|---|---|
| Total (n=248) | <60 (n=67) | ≥60 (n=181) | p | |
| Age (years) | 66.86 (12.6) | 74.9 (9.3) | 63.87 (12.4) | <0.001 |
| Gender (females) (%) | 25 | 31 | 23 | 0.160 |
| Hypertension (%) | 73 | 90 | 66 | <0.001 |
| Active smokers (%) | 38 | 18 | 45 | <0.001 |
| Diabetes mellitus (%) | 41 | 54 | 36 | 0.010 |
| Cardiovascular disease (%) | 8 | 13 | 6 | 0.060 |
| COPD (%) | 13 | 13 | 13 | 0.880 |
| History of IC (%) | 38 | 46 | 35 | 0.120 |
| Peripheral vasc. disease (%) | 8 | 10 | 8 | 0.500 |
| Dyslipidemia (%) | 66 | 70 | 65 | 0.460 |
| History of HF (%) | 6 | 13 | 4 | 0.006 |
| Chronic atrial fibrillation (%) | 13 | 16 | 12 | 0.320 |
| Anemia at admission (%) | 25 | 48 | 16 | <0.001 |
| BMI (kg/m2) | 28.9 (4.4) | 29.5 (4.7) | 28.7 (4.3) | 0.200 |
| Albumin/Cr(o) ratio >30mg/g | 19.3 | 39 | 11.9 | <0.001 |
| Escale TIMIa | 3 (3–4) | 4 (3–5) | 3 (3–4) | 0.030 |
| Albumin/Cr(o)a | 10.8 (6.3–23.8) | 17.5 (10–74.6) | 9.2 (5.8–18.5) | <0.001 |
| Total cholesterol (mg/dl) | 157.4 (35.8) | 152.5 (32.5) | 159.1 (36.8) | 0.210 |
| LDL-C (mg/dl) | 95.8 (30.4) | 89.7 (25.4) | 97.9 (31.7) | 0.070 |
| Triglycerides (mg/dl) | 138.7 (60.6) | 1487 (64.1) | 135.1 (59.2) | 0.130 |
| Hemoglobin (g/dl) | 13.2 (1.7) | 12.3 (1.8) | 13.5 (1.6) | <0.001 |
| CRP ultrasensitive (mg/l) | 18.8 (28.9) | 23.6 (35.8) | 17.1 (25.7) | 0.130 |
| Troponin I (ng/dl) | 1.49 (7.3) | 1.12 (2.6) | 1.6 (8.4) | 0.700 |
| BNP (pg/ml) | 190.8 (342.3) | 357.3 (447.4) | 128.7 (269.9) | <0.001 |
| LVEF (%) (n=228) | 57.4 (10.1) | 54 (12.1) | 58.6 (8.9) | 0.002 |
| Oral anticoagulation | 8.5 | 14.9 | 6.1 | 0.030 |
| ASA | 93.5 | 89.6 | 95 | 0.120 |
| Double antiaggregation oral (%) | 78 | 70 | 81 | 0.060 |
| ACEI/ARAII (%) | 77 | 76 | 77 | 0.840 |
| Statins (%) | 93 | 90 | 94 | 0.240 |
| Betablocker (%) | 85 | 87 | 84 | 0.620 |
The values of continuous variables are expressed as mean and standard deviation (SD).
ASA: acetylsalicylic acid; albumin/Cr(o): albumin/creatinine in urine; BNP: cerebral natriuretic peptide; IC: ischemic heart disease; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; HF: heart failure; ACEI/ARAII: angiotensin converting enzyme inhibitors/angiotensin II receptor antagonists; BMI: body mass index; LDL-C: low density lipoprotein; CRP: C-reactive protein; eGFR: estimated glomerular filtration rate; TIMI: thrombolysis in myocardial infarction.
Significant coronary stenosis was observed in 83.5% (n=207), with no difference in the number of vessels affected between both groups. A revascularization procedure was performed in 179 patients (86.5%) with coronary lesions; the procedure was applied in a lower percentage in CKD patients and the percent of patients with revascularization was decreased with the degree of renal failure: (93.2% for ≥90ml/min/1.73m2 and 72.7% for <45ml/min/1.73m2; showing a significant trend p>0.007). The median time to catheterization was 4 days and revascularization was 5 days. Percutaneous coronary intervention (PCI) was the most frequent procedure, about 90% of cases, and it was similar in both groups. The type of invasive strategy adopted (urgent, precocious or elective) was similar in both groups (Table 2). Among the 28 cases (13.5%) that were not revascularized, it was more frequent to have CKD (42.9 vs. 22.7%; p=0.023), acute renal damage (21.4 vs. 8%; p=0.026) and anemia (39.3 vs. 21%; p=0.034).
Findings in relation to coronary angiography and revascularization procedures.
| eGFR(ml/min/1.73m2) | ||||
|---|---|---|---|---|
| Total | <60 | ≥60 | p | |
| Days until catheterization, median (ICR) | 4 (1–6) | 5 (2–7) | 4 (1–6) | 0.06 |
| Days until PCI [median (IAR)] | 5 (2–8) | 7 (2–10) | 4 (2–7) | 0.07 |
| Type of invasive strategy (%): | ||||
| Urgent (<1 day) | 26 | 22 | 27 | 0.45 |
| Early (1–3 days) | 20 | 15 | 22 | 0.21 |
| Scheduled (>3 days) | 54 | 63 | 51 | 0.10 |
| Results of coronary angiography (%): | ||||
| without significant estenosis | 16 | 21 | 15 | 0.34 |
| 1 vessel | 33 | 33 | 33 | 0.89 |
| 2 vessels | 22 | 16 | 24 | 0.22 |
| 3 vessels | 29 | 30 | 28 | 0.86 |
| Isolated involvement of LCT | 2 | 2 | 3 | 0.77 |
| Affected the AD | 25 | 27 | 24 | 0.71 |
| Revascularization n (n=207) (%): | 86 | 77 | 90 | 0.02 |
| PCI (n=157) (%) | 88 | 90 | 87 | 0.57 |
| ACBP (n=22) (%) | 12 | 10 | 13 | 0.57 |
| Complete revascularization PCI (%) | 56 | 49 | 58 | 0.30 |
| PCI with stent (n=157) (%) | 94 | 89 | 96 | 0.13 |
| PCI with stent with active drug (n=147) (%) | 63 | 62 | 63 | 0.90 |
ICR: interquartile range; ACBP: aortocoronary bypass surgery; AD: anterior descending coronary artery; PCI: percutaneous coronary intervention; LCT: left common trunk; eGFR: estimated glomerular filtration rate.
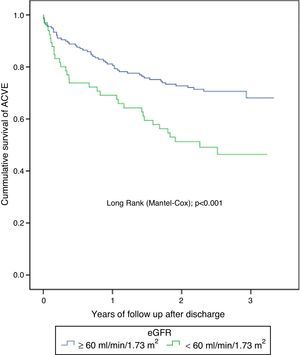
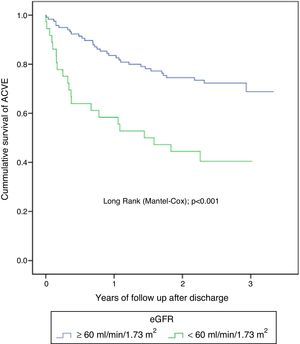
The median follow-up after discharge was 2.22 years. There were 84 ACVE and 9 deaths due to cardiovascular causes (3.6%). The total number of re-admission for cardiovascular causes and separated by its individual components was more frequent in the CKD group (Table 3). The cumulative probability of presenting ACVE during the 3-year follow-up was more frequent among patients with CKD (49.3 vs. 28.2%; p=0.001) (Fig. 2). Revascularization did not modify the ratio of renal failure to the combined event (Pinteraction=0.88) and (Pinteraction=0.12) specifically for PCI (Fig. 3). Non-cardiovascular death was confirmed in 20 patients (8.1%); there were 14.9% vs. 5.5% (p=0.02) for the groups with and without CKD, respectively.
Adverse events during 3 years follow-up according to eGFR.
| eGFR (ml/min/1.73m2) | ||||
|---|---|---|---|---|
| Total | <60 | ≥60 | p | |
| Cardiovascular death | 9 (3.6) | 4 (6) | 5 (3) | 0.230 |
| Total deaths | 29 (12) | 14 (21) | 15 (8) | 0.006 |
| Cardiovascular re-admission | 75 (30) | 29 (43) | 46 (25) | 0.007 |
| Acute coronary syndrome | 53 (21) | 18 (27) | 35 (19) | 0.200 |
| Heart failure | 16 (6) | 9 (13) | 7 (4) | 0.006 |
| Stroke | 6 (2) | 2 (3) | 4 (2) | 0.720 |
Data are expressed in number of cases and (%).
eGFR: estimated glomerular filtration rate.
The estimated risk for the combined event in patients with an eGFR<60ml/min/1.73m2 was two fold increased as compared with cases with ≥60ml/min/1.73m2 even in the adjusted model (HR: 1.94; 95% CI: 1.12–3.27; p=0.012) (Table 4). Evaluation of the different degrees of renal function revealed a significant linear trend (p<0.0001) between the reduction of the eGFR and the risk of ACVE. In the adjusted analysis, as compared to patients with normal renal function, the risk was 1.61 if the eGFR (ml/min/1.73m2) was between 60 and 89; 2.60 if eGFR was between 45 and 59 and 2.95 if it was <45 (Table 5).
Unadjusted and adjusted risk rates of 3-year combined event according to eGFR.
| eGFR (ml/min/1.73m2) | HR (95% CI) | p | HR adjusteda (95% CI) | p |
|---|---|---|---|---|
| <60 vs. ≥60 | 2.45 (1.47–4.08) | 0.001 | 1.94 (1.12–3.27) | 0.01 |
| ≥90 (n=87) (reference) | ||||
| 60–89 (n=94) | 1.71 (0.97–3.02) | 0.06 | 1.61 (0.85–3.08) | 0.15 |
| 45–59 (n=40) | 2.41 (1.26–4.59) | 0.008 | 2.6 (1.21–5.59) | 0.01 |
| <45 (n=27) | 3.42 (1.74–6.74) | <0.0001 | 2.95 (1.25–6.97) | 0.01 |
HR: hazard ratio; CI: confidence interval; eGFR: estimated glomerular filtration rate.
Adjusted for: history of cardiovascular disease, chronic obstructive pulmonary disease, history of ischemic heart disease or known coronary stenosis, history of heart failure, presence of atrial fibrillation and anemia, TIMI scale (thrombolysis in myocardial infarction), acute renal damage, extent of coronary disease and treatment, including revascularization during admission and optimal medical treatment at discharge.
Independent predictors of ACVE after 3 years.
| Predictors | HR (95% CI) | p |
|---|---|---|
| CKD | 1.66 (1.05–2.61) | 0.03 |
| Diabetes mellitus | 2.1 (1.35–3.28) | 0.001 |
| Bleeding during admission | 2.42 (1.06–5.51) | 0.04 |
| History of heart failure | 2.48 (1.21–5.09) | 0.01 |
ACVE: adverse cardiovascular events; CKD: chronic kidney disease; HR: hazard ratio; CI: confidence interval.
Multivariate analysis confirmed that the baseline CKD was an independent predictor of combined event risk (HR: 1.66; 95% CI: 1.05–2.61; p=0.03), comparable to the value associated with diabetes mellitus. In 82.7% of the cases, renal function remained stable during hospitalization. The correlation between baseline values and creatinine clearance showed a Spearman coefficient of ρ=0.77; p<0.0001.
DiscussionIn our population of patients with NSTE-ACS with invasive treatment, there were a 27% of cases with CKD on admission. This is similar to the one observed by others based on patients treated with catheterization15,24 but lower than that of large NSTE-ACS registries.4 We have observed a higher rate of ACVE in cases with CKD patients, with a long-term absolute increase in risk of 21%.
As in other studies5,15,24 there is a reduced frequency of active smoking in the CKD group. This figure may be related to the higher number of hospital admissions for ischemic heart disease and heart failure, that may result in correction of modifiable risk factors. In addition, compared with those with normal renal function, patients with CKD are older and have a poor cardiovascular profile, with a higher prevalence of hypertension and diabetes. However, the increased risk of ACVE observed in patients with renal failure is maintained even after adjusting for differences in baseline clinical characteristics. This means that comorbidity accounts for only part of the risk observed in CKD. Analysis of the relative contribution of age, conventional risk factors and CKD to the combined event at follow-up revealed that the risk associated with CKD was comparable to that associated with diabetes. This finding is in agreement with recent meta-analysis25 and studies that evaluate only short-term and hospital events14 and highlight the role of CKD as a risk factor.
In the last decades, the prognosis in NSTE-ACS has improved.9 One question is whether the subgroup of patients with CKD receives optimal management. Recent studies examining the trend in OMT adoption and revascularization strategies confirm less application of these procedures in CKD,4,24,25 even in well-regulated clinical trials.5 We also observed that in the CKD group, revascularization was less frequent, although it reached higher percentages than those reported in the mentioned studies. One explanation for this may be that patients with CKD have been poorly represented or excluded from pivotal clinical trials assessing the advances in the management strategy of NSTE-ACS.26–30 Therefore, it is largely unknown how these optimal strategies improve the prognosis of these patients and whether their use leads to a prolonged benefit over time at all levels of CKD severity. Consistent with observations from large registries,11,23,31 our results show that, even after revascularization, CKD maintains a higher risk of long-term ACVE. This lack of overall benefit is probably due to the greater complexity of coronary stenosis and the increase in complications following the procedure associated with PCI.15,32
There are few studies evaluating the specific influence of CKD on outcomes in NSTE-ACS patients managed exclusively with an invasive strategy. In some, as in a recent retrospective analysis of the ACTION registry, it has been shown that CKD is a potent predictor of hospital mortality in patients with NSTE-ACS treated with PCI.15 In the study by Bonello et al.,16 also retrospective including NCTE-ACS and NSTE-ACS, it was observed that, despite an optimal treatment, the prognosis of patients with CKD was worse than those with normal renal function. Previously, Mueller et al.33 investigated the association between baseline renal function and total mortality following an NSTE-ACS in a cohort of patients treated predominantly with very early PCI (median 5h). After adjustment, CKD remained a predictor of long-term mortality (HR: 2.6; 95% CI: 1.5–4.5).
Our study has some characteristics of its own; the data are extracted from situations of usual clinical practice, in which long-term cardiovascular events are analyzed. It includes only patients with NSTE-ACS who underwent coronary angiography to separate the effect of CKD and the result of underuse of therapies has proven beneficial effect and, we excluded dialysis patients to avoid bias of the greatest impairment of renal function on the ACVE.16 Despite the high rate of revascularization, the absence of differences in the frequency of OMT prescription at discharge and in the use of drug-eluting stents there was a significant and gradual association between the reduction of GFR and the long-term risk of the combined event.
Our results and those obtained of others,12,34 indicate that factors related to renal failure contribute to a worse prognosis after NSTE-ACS. Recent investigations have shown that CKD predisposes to a reduced reserve of coronary blood flow which predicts events independently of the extent of coronary affected.35 This may explain why even with no differences in the severity of coronary disease between the two groups, CKD patients presented a higher risk of long-term ACVE. In addition, these patients had a significant increase in albuminuria compared to those with normal renal function. Epidemiological studies have shown a gradual relationship between degree of albuminuria and ACVE that is independent of traditional cardiovascular risk factors.1,36 Other incidents such as acute kidney damage21 and anemia,37 which are associated to adverse cardiovascular events in CKD, were also more frequent in these patients.
Our findings support the consideration of CKD as an independent predictor of cardiovascular complications following an NSTE-ACS and indicate that specific factors related to renal dysfunction influence the prognosis of these patients.
LimitationsThe CKD was defined by the eGFR calculated with the first value of serum creatinine determined in the hospital. Diagnosis of a chronic process requires the demonstration of kidney damage or decreased kidney function for at least 3 months. However, we consider that our results may be compared with other studies that also use the value of baseline serum creatinine at admission to classify the CKD group. It is an observational study, with data extracted from a single center, and the indication of an invasive strategy was at the discretion of the responsible physician, which may have a bias. The eGFR may have varied during the admission period; however, we analyzed its stability and the relation between the values at admission and discharge showed a good correlation. Finally, the registry was not specifically designed to assess the risks and benefits of an invasive strategy in CKD patients with NSTE-ACS.
ConclusionThe CKD is common in patients with NSTE-ACS treated with an invasive therapy. Our results indicate that in this clinical setting the decreased renal function is an independent predictor of long-term cardiovascular adverse events and that its negative effect on prognosis is maintained despite the modality of treatment.
Patients with CKD and NSTE-ACS have been poorly represented or excluded from clinical trials aiming to assess advanced strategies in the management of NSTE-ACS, so definitive data are missing in this specific population. The effects on the long-term prognosis of an invasive strategy, with and without revascularization, in this subgroup of patients are largely unknown.
What is new?Our study analyzes real-life data from consecutive patients with NSTE-ACS, some of which would have been excluded from the large randomized trials, and evaluates the specific use of an invasive therapy. Our results confirm that CKD has negative long-term prognostic impact and provides a reference for rates of cardiovascular adverse events in routine clinical practice.
The authors have no conflicts of interest to declare.
Please cite this article as: Roldán Torres I, Salvador Mercader I, Cabadés Rumbeu C, Díez Gil JL, Ferrando Cervelló J, Monteagudo Viana M, et al. Pronóstico a largo plazo de la enfermedad renal crónica en el síndrome coronario agudo sin elevación del segmento ST tratado con estrategia invasiva. Nefrologia. 2017;37:276–284.