To describe the clinical characteristics, the reasons for initiating therapy and the effects of treatment in the initial phase of evolocumab availability in the Nephrology Units of Spain.
Material and MethodsRetrospective, observational and multicentric study that included patients initiating treatment with evolocumab (from February 2016 to August 2018), in 15 Nephrology Units in Spain. The demographic and clinical characteristics of the patients, the lipid lowering treatment and the evolution of the lipid profiles between 24 weeks pre-initiation and 12±4 weeks post-initiation of evolocumab were reviewed.
Results60 patients were enrolled: 53.3% women; mean (SD) age, 56.9 (12.8) years, 45.0% with familial hypercholesterolemia (FH) (5.0% homozygous and 40.0% heterozygous) and 65.0% with atherosclerotic cardiovascular disease. The mean (SD) eGFR was 62.6 (30.0) ml/min/1.73m2 (51.7% of patients had eGFR <60ml/min/1.73m2 [CKD stage>2]), 50.0% had proteinuria (>300mg/g) and 10.0% had nephrotic syndrome. Other CV risk factors were hypertension (75.0%), diabetes (25.0%), and smoking (21.7%). A 40.0% of patients were statin intolerant. At evolocumab initiation, 41.7% of patients were on a high intensity statin, 18.3% on moderate intensity statin and 50.0% were receiving ezetimibe. Mean (SD) LDL-c at evolocumab initiation was 179.7 (62.9) mg/dL (53.4% of patients with LDL-c ≥160mg/dL and 29.3% ≥190mg/dL). After 12 weeks, evolocumab resulted in LDL-c reductions of 60.1%. At week 12, 90.0% of patients reached LDL-c levels <100mg/dL, 70.0% <70mg/dL, and 55.0% <55mg/dL, while mean eGFR levels and statin use remained stable.
ConclusionIn Nephrology Units of Spain, evolocumab was predominantly prescribed in patients with FH, chronic renal disease (CRD>2) and secondary prevention, with LDL-c levels above those recommended by the guidelines. Evolocumab used in clinical practice significantly reduced the LDL-c levels in all patients included in the study.
Describir las características clínicas de los pacientes tratados con evolocumab, las razones del inicio de la terapia y los efectos del tratamiento en la fase inicial de disponibilidad de evolocumab en las unidades de Nefrología de España.
Material y MétodosEstudio retrospectivo, observacional, y multicéntrico que incluye los pacientes que iniciaron tratamiento con evolocumab (desde febrero 2016 a agosto 2018), en 15 unidades de Nefrología en España. Se revisaron las características demográficas y clínicas de los pacientes, el tratamiento hipolipemiante y la evolución de los perfiles lipídicos entre 24 semanas antes y 12±4 semanas después del inicio de evolocumab.
ResultadosSe incluyeron 60 pacientes: 53,3% mujeres, edad media (DE) de 56,9 (12,8) años, 45,0% con hipercolesterolemia familiar (HF) (5,0% homocigota y 40,0% heterocigota), y 65,0% con enfermedad cardiovascular aterosclerótica (ECVA) previa. El filtrado glomerular estimado (FGe) medio fue 62,6 (30,0) ml/min/1,73m2 (51,7% pacientes con FGe<60ml/min/1,73m2 [ERC estadio >2]), 50,0% proteinuria (>300mg/g) y 10,0% síndrome nefrótico. Otros factores de riesgo CV fueron: hipertensión (75,0%), diabetes mellitus (25,0%) y hábito tabáquico (21,7%). El 40,0% eran intolerantes a estatinas. Al inicio de evolocumab, el 41,7% tomaban estatinas de alta intensidad, el 18,3% estatinas de moderada intensidad y el 50,0% ezetimiba. Los niveles medios (DE) de c-LDL al inicio de evolocumab fueron de 179,7 (62,9) mg/dL (53,4% pacientes con c-LDL≥160mg/dL y 29,3% ≥190mg/dL). Después de 12 semanas del tratamiento con evolocumab se observó una reducción de los niveles de c-LDL del 60,1%. A la semana 12, el 90,0% de pacientes alcanzó niveles c-LDL <100mg/dL, 70,0% <70mg/dL y 55,0% <55mg/dL, mientras que el FGe medio y el uso de estatinas se mantuvieron estables.
ConclusionesEn las unidades de nefrología de España, evolocumab se ha prescrito principalmente en pacientes con HF, enfermedad renal crónica (ERC>2) y prevención secundaria, con niveles de c-LDL muy por encima de los recomendados por las guías. Evolocumab utilizado en práctica clínica, redujo significativamente los niveles de c-LDL en todos los pacientes incluidos en el estudio.
The global prevalence of chronic kidney disease (CKD) in Spain (according to the EPIRCE study) in stages 3–5 is 3.3% in 40–64 years old patients and 21.4% in older than 64 years1. This prevalence is even higher according to data from 2018, reaching one in 7 adults2. The results of the NEFRONA study in patients with CKD show a high prevalence of cardiovascular (CV) risk factors, observing a significant association of these factors with the progression of atheromatosis: diabetes, dyslipidemia, smoking and hypertension3. Several studies have shown that patients in the early stages of CKD already have a high prevalence of atherosclerotic plaques compared to individuals with normal renal function4,5. In addition, Bermúdez-López et al.6 showed that non-diabetic patients with CKD present a hidden profile of pro-atherogenic lipoproteins.
CV disease is the main cause of premature morbidity and mortality in CKD patients, even during the early stages of CKD3,7–9. CKD patients show a higher incidence of coronary artery disease, myocardial infarction, congestive heart failure, cerebrovascular disease, peripheral arterial disease, and sudden cardiac death10,11. Among the CV risk factors presented by these patients, dyslipidemia is one factor that favors the progression of CKD and increases atherosclerosis and its complications8,9. Adequate control of dyslipidemia contributes to reducing the high CV morbidity and mortality of these patients12.
Treatment with poner statins en minuscula has been shown to improve the prognosis of atherosclerotic cardiovascular disease (ACD) in the general population. However, in patients with CKD, the evidence is scarce due to the few clinical trials carried out in this population. Analysis of retrospective data from a cohort of CKD patients showed that statin treatment slowed the progression of kidney failure and reduced all-cause mortality, but only in early CKD stages. In the 2 studies conducted in patients with CKD stage 5 on dialysis (AURORA and 4D), it was not observed that treatment with statin reduced cardiovascular mortality. The SHARP study showed the benefit of treatment of statin associated with ezetimibe in patients with advanced CKD not on dialysis13. Finally, the results of a systematic review and meta-analysis of randomized clinical trials showed that statins reduce mortality and cardiovascular events in people in early stages of CKD14.
Conversely, in epidemiological studies performed in Spain, it is noticeable the low percentage of patients with CKD receiving statins and maintaining adequate LDL-C levels according to CV risk and guidelines recommendations. In the MERENA study, which included patients with CKD stages 3 and 4, only 34.8% of the patients had LDL-c levels <100mg/dl as recommended by the guidelines and only 54.7% were on statins and this was observed at the time of initiation of the present study. This suggests that the CV risk of patients with CKD could be underestimated and the objectives proposed by the different clinical guidelines are not obtained with the current available treatments15.
Evolocumab is a fully human IgG2 monoclonal antibody that selectively binds to proprotein convertase subtilisin/kexin type 9 (PCSK9), increasing hepatic levels of the low-density lipoprotein receptor, leading to a decrease in serum LDL-C levels. In Spain, the cost of evolocumab has been covered by the National Health System since February 1, 2016 for the use in patients with heterozygous or homozygous familial hypercholesterolemia (FH) or patients with previous cardiovascular events who maintain LDL-C levels ≥100mg/dl.
In clinical trials phase 2 and 3, evolocumab has been shown to produce a mean decrease in LDL-C levels of more than 60%, regardless of the baseline lipid-lowering treatment16–20. Likewise, it has been observed that in patients diagnosed with FH and in chronic treatment with LDL-apheresis, treatment with evolocumab reduces LDL-c levels more effectively than LDL-apheresis21.
Hospital Nephrology Departments in Spain are responsible for the clinical care of patients with CKD, the treatment of its complications and the control of associated CV risk factors, paying special attention to dyslipidemia.
The main objective of this systematic clinical record review study was to describe the baseline clinical characteristics of the former patients treated with evolocumab in Hospital Nephrology Departments in Spain. As secondary objectives, we analyzed the therapeutic management of these patients with hyperlipidemia during the first weeks of treatment with evolocumab, as well as the reasons for starting this new therapy.
MethodsStudy designObservational, retrospective and multicenter study. The medical records of patients receiving evolocumab (140mg/14 days) as part of the usual clinical treatment dyslipidemia in Spanish nephrology outpatient clinics were reviewed. Patients included were: age ≥18 years who started treatment with evolocumab prescribed by a physician from a nephrology department (regardless of the study protocol) between February 1, 2016 and August 31, 2018, who had received at least one dose of evolocumab, with at least one LDL-C measurement and one estimated glomerular filtration rate (eGFR) within 24 weeks prior to starting evolocumab. Those patients who had received treatment with a PCSK9 inhibitor in the previous 24 weeks and/or had participated in a clinical study during the study period, that is, 24 weeks before starting treatment with evolocumab or up to 12 weeks after its initiation, were excluded from the present study.
Nephrology Departments from 15 hospitals distributed in different geographical areas throughout Spain participated in the study.
The study protocol was approved by the Ethics Committee of each center and all patients signed an informed consent for the use of their data anonymously.
Clinical data were collected during the 24 weeks prior to the initiation of the treatment with evolocumab and up to 12±4 weeks after its onset. Baseline values were considered to be the latest available analytical parameters measured within the 24 weeks prior to starting treatment with evolocumab.
The following analytical parameters were collected, whenever they were available in the clinical history: lipid profile (LDL-C, HDL-C, total cholesterol, triglycerides), eGFR according to baseline MDRD and post-baseline Last Observation Carried Forward (LOCF).
Post-baseline LOCF was defined as the last available value after initiation of evolocumab. Given that it is a retrospective observational study, not all patients had laboratory tests after starting treatment with evolocumab, within the 12+4 weeks after it was initiated.
The following sociodemographic and clinical variables were recorded at the initiation of treatment with evolocumab: age, sex, employment status, weight, height, body mass index, family CV history (CVD, ischemic heart disease, stroke, peripheral arterial disease, death due to cardiovascular event), personal CV history (heart failure, cerebrovascular disease, transient ischemic attack, carotid arteriosclerotic disease, peripheral arterial disease, angina, myocardial infarction), personal history of dyslipidemia (FH, familial combined hyperlipidemia, other types of hypercholesterolemia and mixed or combined hyperlipidemia), diabetes mellitus, hypertension, history of diabetic nephropathy, CKD and smoking.
The achievement of therapeutic objectives set by the ESC/EAS 2016 hyperlipidemia management clinical guidelines22 (as described in the protocol) and in a subsequent analysis following the recent ESC/EAS 2019 guidelines23 were also analyzed.
Likewise, the use of other lipid-lowering treatments, the presence of intolerance to statins before starting treatment with evolocumab and all lipid-lowering treatments during the subsequent 12 weeks were recorded. Statin intolerance was defined as: intolerance to a first statin at the maximum tolerated dose and to a second statin at any dose with an adverse effect attributed to the drug and resolved on withdrawal.
Objectives and variables of the studyThe main objective was to describe the baseline clinical characteristics (baseline LDL-C levels and estimated glomerular filtration rate) in the former group of patients treated with evolocumab in hospital nephrology departments in Spain.
As secondary objectives, we analyzed, other clinical features of the patients participating in the study (including lipid profile, demographic variables, personal and family medical history), and the type of the clinical management after starting treatment with evolocumab. In addition, the clinical reasons for starting this therapeutic tool were analyzed.
As an exploratory objective, the circuits of the patients referral were also analyzed.
Statistical analysisA descriptive statistical analysis was performed for all variables. Quantitative variables were described as measures of central tendency and dispersion (mean, standard deviation [SD], minimum, 25% quartile [Q1], median, 75% quartile [Q3], and maximum for nonparametric variables). For the qualitative variables there were used frequency tables and percentages of the total evaluable responses. In all cases, the confidence intervals applied were 95% (95% CI).
The decrease in LDL-C after treatment with evolocumab was compared (exploratory testing) in patients with and without FH, with and without nephrotic syndrome, and with eGFR ≥ and <60ml/min/1.73m2. Differences between groups were analyzed using parametric tests if the sample followed a normal distribution (Student’s t-test) and non-parametric tests if it did not follow a normal distribution (Mann–Whithney test).
Statistical significance was considered at values less than 0.05.
All statistical analyzes were performed using the statistical package SAS® System for Windows version 9.4.
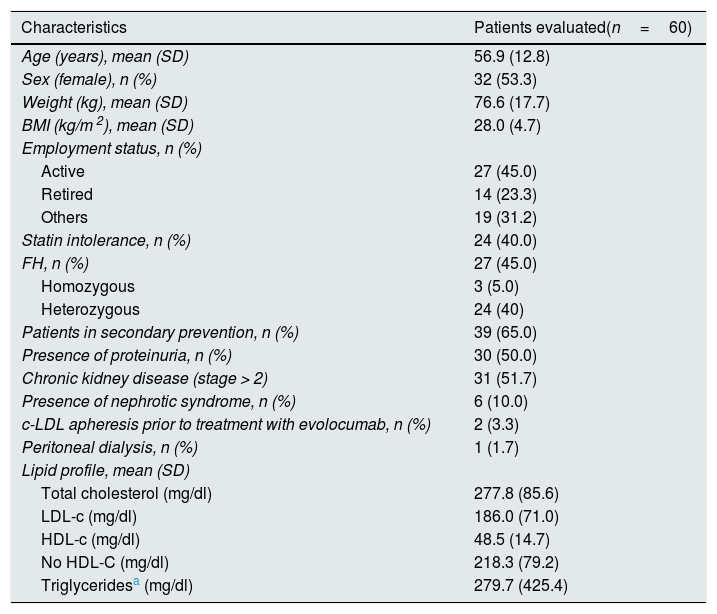
ResultsBaseline characteristicsA total of 60 patients with a mean age of 56.9 (12.8) years were included in the study. The baseline sociodemographic and clinical characteristics are shown in Table 1.
Baseline sociodemographic and clinical characteristics.
| Characteristics | Patients evaluated(n=60) |
|---|---|
| Age (years), mean (SD) | 56.9 (12.8) |
| Sex (female), n (%) | 32 (53.3) |
| Weight (kg), mean (SD) | 76.6 (17.7) |
| BMI (kg/m 2), mean (SD) | 28.0 (4.7) |
| Employment status, n (%) | |
| Active | 27 (45.0) |
| Retired | 14 (23.3) |
| Others | 19 (31.2) |
| Statin intolerance, n (%) | 24 (40.0) |
| FH, n (%) | 27 (45.0) |
| Homozygous | 3 (5.0) |
| Heterozygous | 24 (40) |
| Patients in secondary prevention, n (%) | 39 (65.0) |
| Presence of proteinuria, n (%) | 30 (50.0) |
| Chronic kidney disease (stage > 2) | 31 (51.7) |
| Presence of nephrotic syndrome, n (%) | 6 (10.0) |
| c-LDL apheresis prior to treatment with evolocumab, n (%) | 2 (3.3) |
| Peritoneal dialysis, n (%) | 1 (1.7) |
| Lipid profile, mean (SD) | |
| Total cholesterol (mg/dl) | 277.8 (85.6) |
| LDL-c (mg/dl) | 186.0 (71.0) |
| HDL-c (mg/dl) | 48.5 (14.7) |
| No HDL-C (mg/dl) | 218.3 (79.2) |
| Triglyceridesa (mg/dl) | 279.7 (425.4) |
SD: standard deviation; FH: familial hypercholesterolemia; BMI: body mass index.
Two patients presented apheresis, with mean values (SD) of 413.5 (71.4) mg/dl total cholesterol; 370.5 (40.3) mg/dl LDL-c; 49.5 (6.4) mg/dl c-HDL; 347.0 (73.5) mg/dl non-HDL-C and 82.0 (42.4) mg/dl triglycerides.
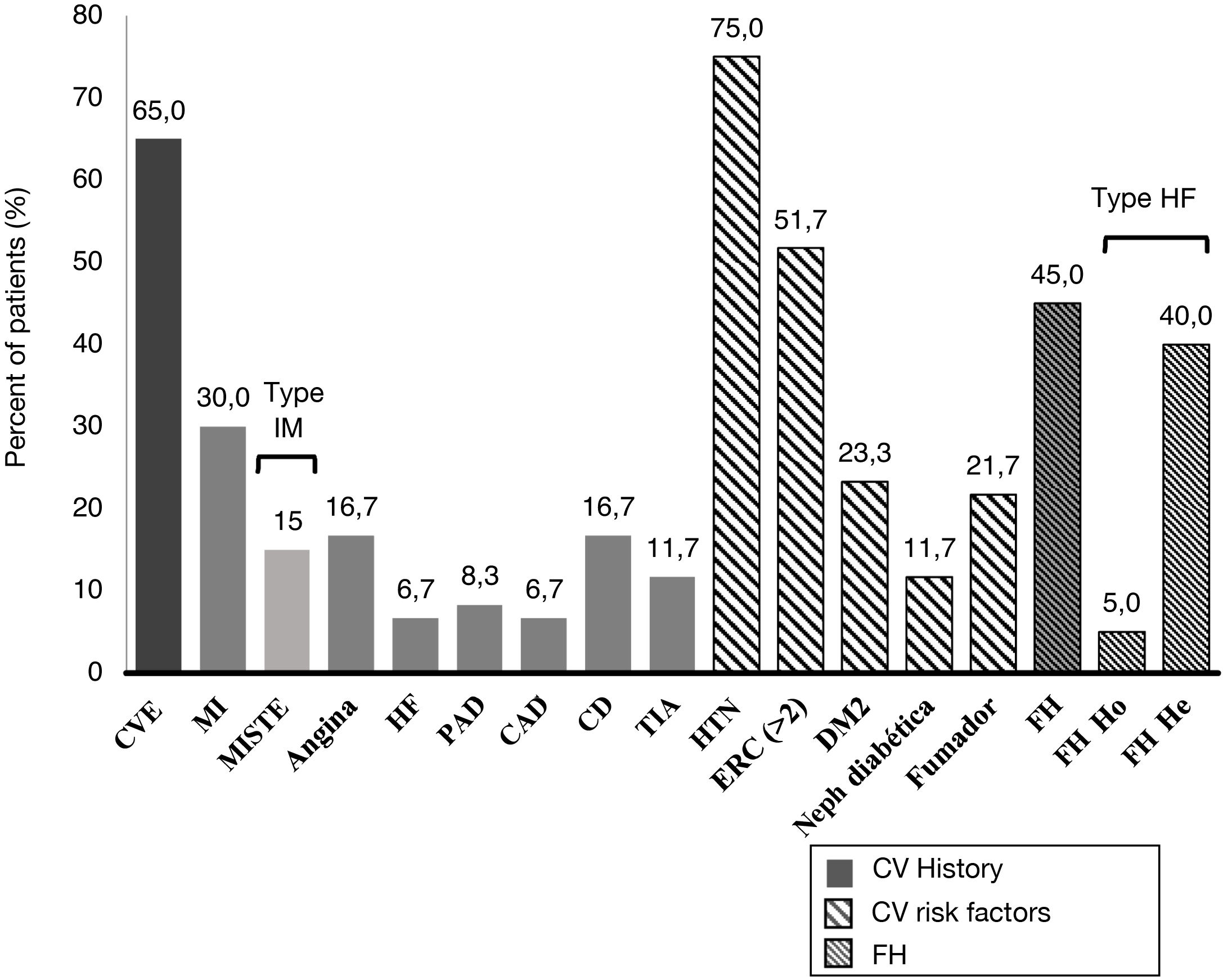
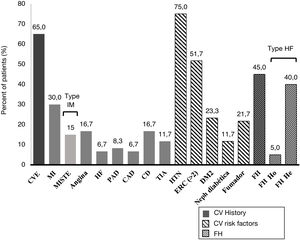
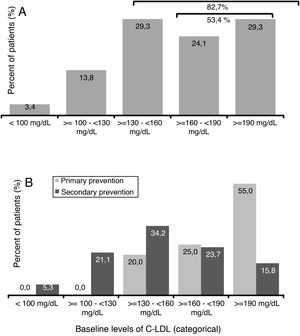
A 45.0% of the patients included in the study were diagnosed with FH (3 of them with homozygous FH), more than half (51.7%) had CKD with stage > 2, and 40.0% were statin intolerant (mainly to atorvastatin [70.8%] followed by pitavastatin [33.3%] and simvastatin [25.0%]), with a median [Q1–Q3] time from detection of intolerance of 1.3 [0.8–5.0] years). A 65.0% of patients had a history of established ACD. Personal history and baseline CV risk factors are shown in Fig. 1.
Cardiovascular clinical history and cardiovascular risk factors.
TIA: transient ischemic attack; CV: cardiovascular; DM2: type 2 diabetes mellitus; CAD: carotid arteriosclerotic disease; PAD: peripheral arterial disease; CD: cerebrovascular disease; CVE: cardiovascular event; He: heterozygous; FH: familial hypercholesterolemia ; Ho: homozygous; HTN: hypertension; HF: heart failure; MI: myocardial infarction; MISTE: myocardial infarction with ST-segment elevation; Neph: nephropathy.
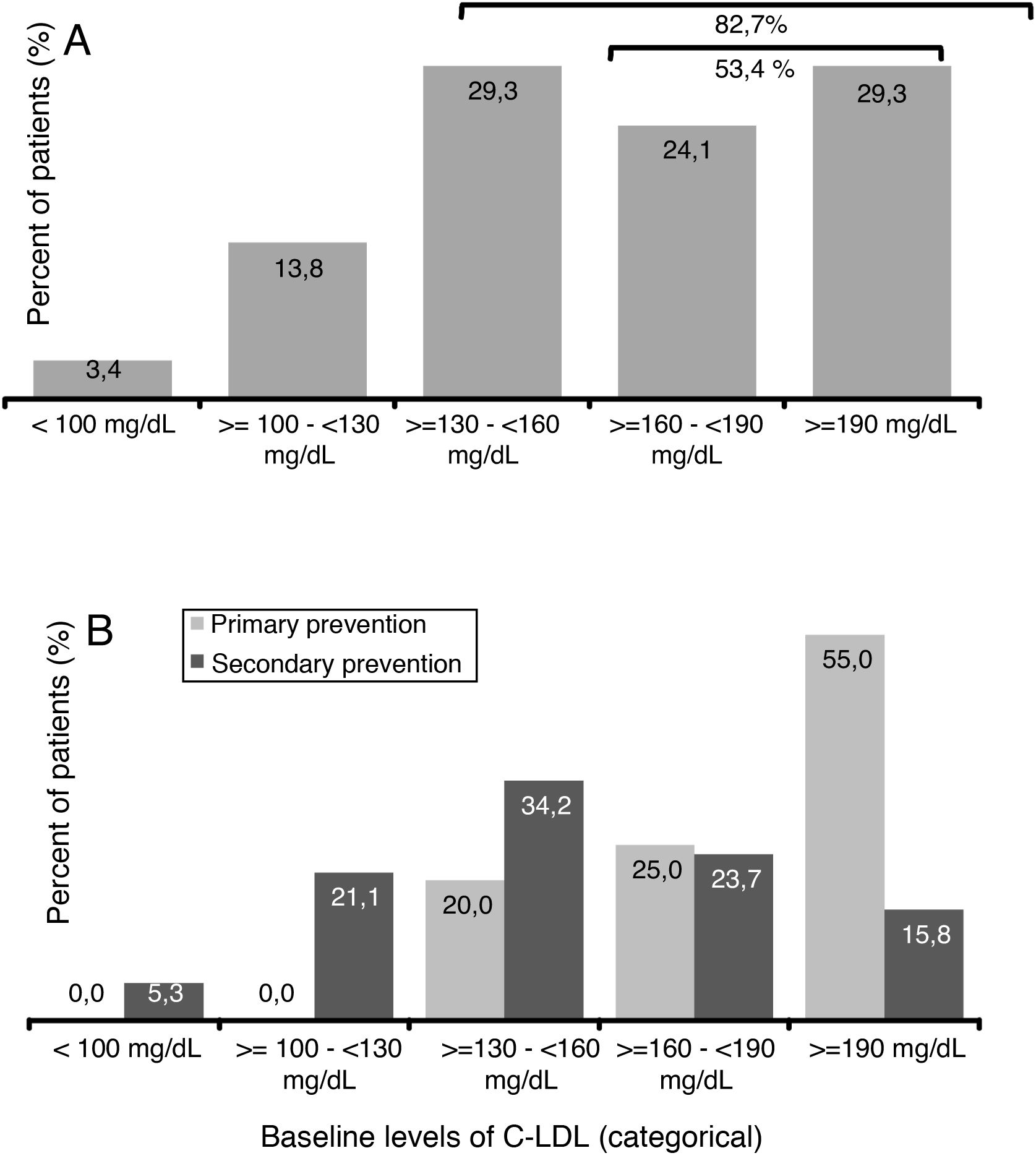
The mean value (SD) of c-LDL before starting treatment with evolocumab was 179.7 (62.9) mg/dl (Table 1). A 29.3% of patients presented levels ≥130 and <160mg/dl, 24.1% had ≥160 and <190mg/dl and 29.3% of patients had levels ≥190mg/dl (Fig. 2A). Among patients in primary prevention, 80% had LDL-C ≥160mg/dl, while in the patients in secondary prevention, 60.6% had baseline levels <160mg/dl (Fig. 2B).
The mean (SD) value of eGFR before starting treatment with evolocumab was 62.6 (30.0) ml/min/1.73m2, with a median (Q1–Q3) of 60.1 (38.9–89.6) ml/min/1.73m2.
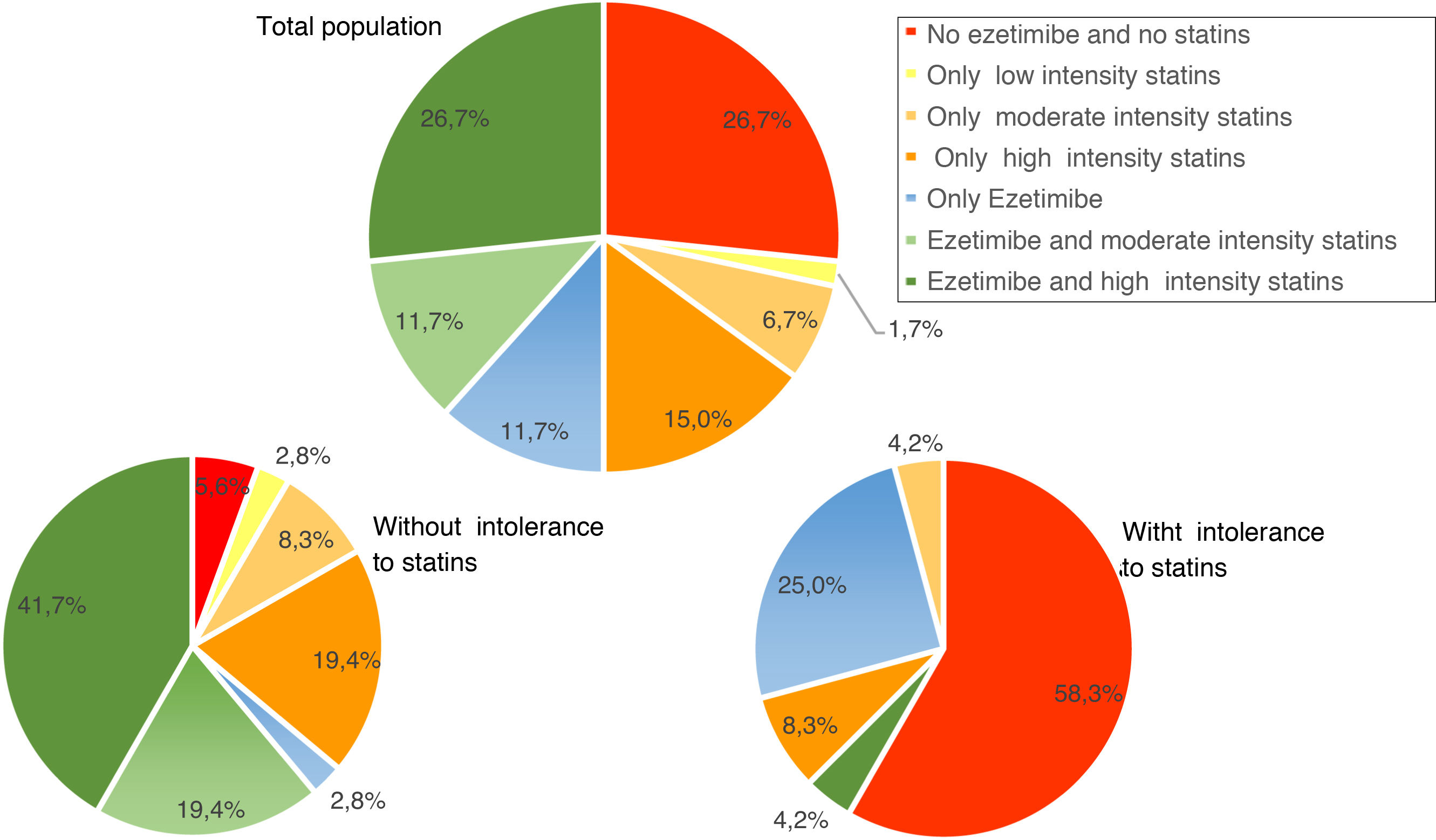
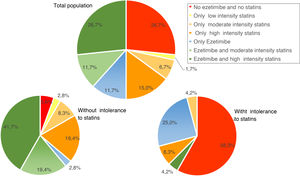
Use of lipid-lowering treatmentsAt baseline, 41.7% of patients were taking high-intensity statins, 50.0% were on ezetimibe, and 38.2% were taking both. The median (Q1–Q3) duration of pre-initiation treatment with evolocumab was 19.1 (7.2–42.9) months for high-intensity statins and 10.2 (3.2–31.1) months in the case of ezetimibe. Fig. 3 shows the percentage of patients treated with ezetimibe and/or statins (according to the intensity of treatment) in the total population and in the population with and without intolerance to statins, before starting treatment with evolocumab.
The clinical reasons to indicate treatment with evolocumab are those indicated in its therapeutic positioning report (IPT24); it was mainly indicated in 65% due to the presence of prevalent CV disease and in 45% of the cases due to the existence of familial hypercholesterolemia, mainly heterozygous.
Treatment with oral lipid-lowering medications (both statins and ezetimibe) remained stable in most patients throughout the study period. Regarding treatment with evolocumab, only one patient discontinued treatment during the 12 weeks after starting with evolocumab due to change of residence to another region.
Evolution of the lipid profile after initiation of evolocumabAfter 12 weeks of treatment with evolocumab LDL-C levels decreased by 60.1% down to mean values of 72.7mg/dl.
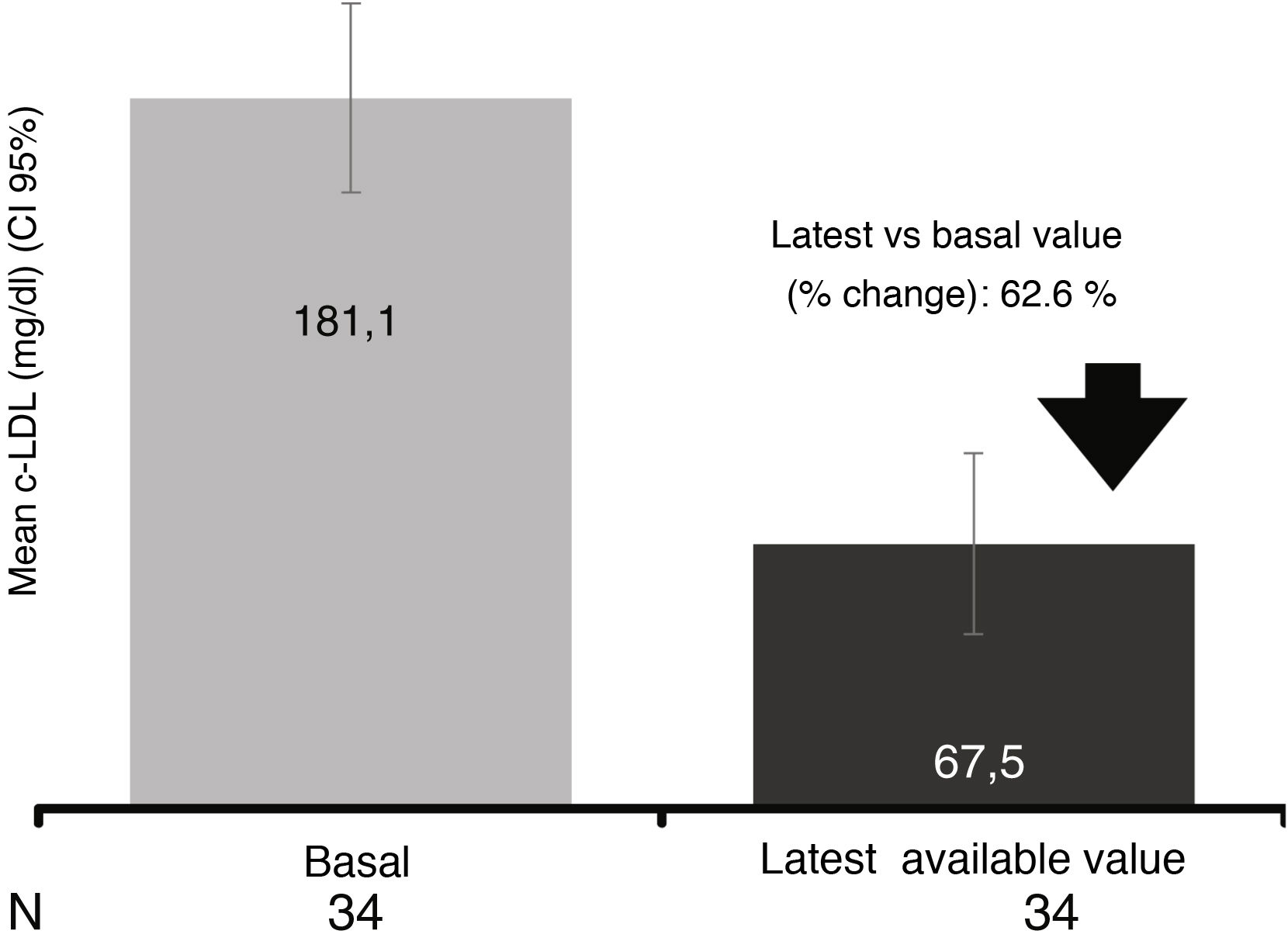
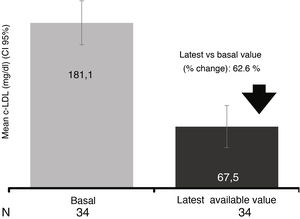
In the 34 patients who had at least one study after initiation of evolocumab treatment (LOCF), a mean reduction of 62.6% was observed, reaching a mean value of 67.5mg/dl (reduction of 62.6 % after a median follow-up of 13.6 weeks) (Fig. 4).
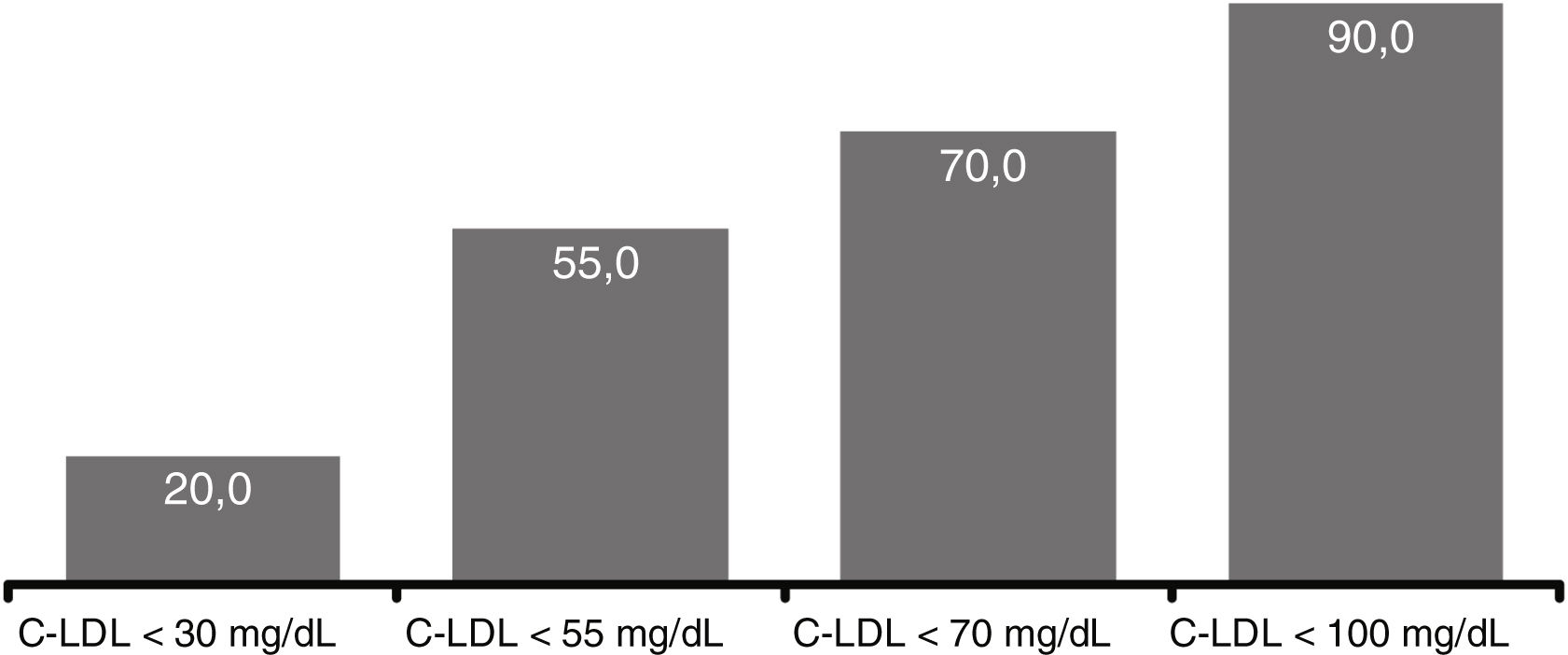
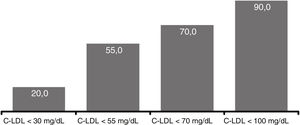
At 12 weeks, 20.0% of patients reached LDL-C levels <30mg/dl, in 55.0% the levels were <55mg/dl, in 70.0% <70mg/dl and 90.0% <100mg/dl) (Fig. 5). There were no significant differences comparing the efficacy of evolocumab in primary/secondary prevention, in different stages of CKD (>2/≤ 2) or in the presence/absence of proteinuria.
Evolocumab treatment did not produce significant changes in HDL-c and triglyceride levels. Mean baseline HDL-C levels were 48.5 (14.7) mg/dl (n=59) and 53.1 (16.1) mg/dl (n=21) in patients with available data at 12 weeks (an increase of 10.5%; p=0.436). Likewise, the basal levels of triglycerides were 279.7 (425.4) mg/dl (n=57), showing a decrease at 12 weeks to 150.6 (94.3) mg/dl (a decrease of 22 .3 %, p=0.249). In the follow-up period, the eGFR remained stable. There was no change in eGFR in patients with baseline measurements, and the latest available value (n=41) (62.8ml/min/1.73m2 and 60.0ml/min/1.73m2, respectively).
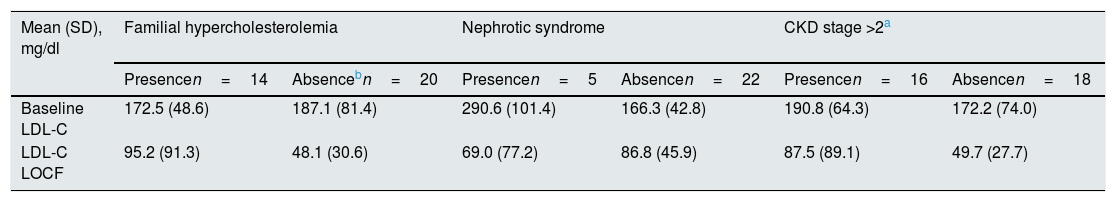
In an exploratory manner, the mean LDL-c values were analyzed according to the presence or absence of FH, nephrotic syndrome or CKD, which are shown in Table 2. The mean reduction in LDL-c levels in patients without FH was significantly higher than in patients with HF (46.7 vs. 73.6%; p=0.005), while no significant differences were observed according to the presence/absence of nephrotic syndrome (58.9 vs. 69.8%; p=0.857) or CKD (62.6 vs. 68.8%; p=0.334).
LDL-C values according to the presence or absence of familial hypercholesterolemia, nephrotic syndrome or chronic kidney disease.
| Mean (SD), mg/dl | Familial hypercholesterolemia | Nephrotic syndrome | CKD stage >2a | |||
|---|---|---|---|---|---|---|
| Presencen=14 | Absencebn=20 | Presencen=5 | Absencen=22 | Presencen=16 | Absencen=18 | |
| Baseline LDL-C | 172.5 (48.6) | 187.1 (81.4) | 290.6 (101.4) | 166.3 (42.8) | 190.8 (64.3) | 172.2 (74.0) |
| LDL-C LOCF | 95.2 (91.3) | 48.1 (30.6) | 69.0 (77.2) | 86.8 (45.9) | 87.5 (89.1) | 49.7 (27.7) |
LOCF: last available LDL-C value after initiation of treatment with evolocumab.
Percent change (presence vs. absence) in: familial hypercholesterolemia (−46.7 vs.−73.6%; p=0.005); nephrotic syndrome (−58.9 vs.−69.8%; p=0.857) and chronic kidney disease (−62.6 vs.−68.8%; p=0.334).
At 12 weeks of treatment with evolocumab, 33.0% (n=6) of CKD stage 3 patients (eGFR 30–59ml/min) reached the target LDL-C level <70mg/dl (defined by the ESC/EAS 2019 guidelines23) and 100.0% of the patients with data available (n=2) with stage 4 CKD (eGFR <30ml/min) reached LDL-c levels <55mg/dl.
Referral circuitsRegarding the management of hyperlipidemia, most of the patients included in the study were already being controlled in nephrology departments (53.3%), 11.7% were detected by primary care physicians, 3.3% by internal medicine, cardiology and endocrinology, respectively, and 6.7% by other hospital departments. In 18.3% of the cases the data was not available.
DiscussionThis study describes the clinical characteristics and therapeutic management of patients with hyperlipidemia followed up in hospital nephrology departments in Spain who started treatment with evolocumab during the first 2 and a half years after its inclusion in the pharmacological guide. The main findings of this analysis are the following: 1) the CKD patients that should receive treatment with evolocumab start with very high LDL-C levels, well above the therapeutic objectives indicated by the current guidelines, 2) the indications for starting treatment with evolocumab were FH, mostly heterozygous, in 45% of cases, and secondary prevention of CV disease in 65%, and 3) treatment with evolocumab reduced LDL-C levels by 60%. Percentage, similar to what was observed in the clinical development program.
The mean levels of c-LDL at the beginning of treatment with evolocumab (180mg/dl), were well above those recommended in the therapeutic positioning report (TPI) of evolocumab (100mg/dl)24, which suggests that in these first patients treated with evolocumab, previous therapeutic options would have failed to achieve LDL-C targets. The prescription of iPCSK9 in Spain is approved in patients with FH or secondary prevention, so for the time being it is not an available therapeutic tool to achieve the "target" recommended by the guidelines in patients with CKD in primary prevention. However, it must be taken into account that since the patients presented in this study were the first patients treated with evolocumab, there was a higher percentage of patients with FH (45%) than would be expected in the daily clinical practice of a nephrology department. Despite the high LDL-C levels at the beginning of the treatment with evolocumab (more than 80% of patients had levels >130mg/dl), these levels decreased abruptly after treatment. In addition, in an exploratory manner due to the interest on certain subpopulations of patients followed in nephrology departments, such as patients with nephrotic syndrome characterized by high levels of LDL25 requiring intense cholesterol reductions, it was observed a significant reduction in LDL-c levels of 70% at 12 weeks of treatment. Taking into account the new objectives defined in the ESC/EAS 2019 guidelines23 and despite the high level of LDL-C in these first patients who started treatment with evolocumab, 3 out of 10 patients with CKD stage 3 reached the therapeutic objectives (defined as c-LDL levels <70mg/dl). In the case of patients with CKD stage 4, the only 2 patients for whom analytical data were available also achieved the therapeutic goal set by the guidelines (defined as LDL-c levels <55mg/dl).
It is important to note that although 40% of the patients had statin intolerance, with 50% receiving ezetimibe, there was a high percentage of patients who were not receiving high-potency statins (58%). This fact may be due to the recommendation of the KDIGO guidelines on lipids26 of 2012, based on the AURORA and 4D studies27,28. In them, the recommended dose of statins in patients with CKD was 20mg of atorvastatin or 10mg of rosuvastatin. However, there are studies published in which patients with CKD and previous CV disease benefit from high doses of statins without adverse side effects29. Thus, statins that have little renal clearance such as atorvastatin and pitavastatin (with renal clearance <5%)30 can be administered to patients with CKD at doses similar to those of the general population.
Similar studies have been performed in Spain on evolocumab in clinical practice in cardiology units and in lipid/internal medicine units31,32. The results are comparable with those obtained in the present study with reductions in LDL-c levels at 12 weeks of treatment of 58% and 56%, respectively26. Being the first group of patients to receive treatment with evolocumab, as in the patients in this study, they had high levels of LDL-C and high cardiovascular risk, which shows that treatment with evolocumab allows the therapeutic goals to be achieved in high risk patients.
The interpretation of the results, and even more so their generalization, must be carried out with caution because the study has some limitations that are inherent to its design. First of all, it is a retrospective and multicenter study, whose main objective was to describe the clinical characteristics and the response to treatment with evolocumab in patients treated in hospital nephrology departments. Therefore, we lack follow-up analytical data for the entire sample, mainly due to the great heterogeneity that exists in the usual clinical practice of the different participating units. Second, the 12-week observation time does not allow to assess the long-term impact of therapy. Likewise, this study has a small sample size, which includes the former patients who received evolocumab in hospital nephrology departments in Spain. These patients did not have an effective alternative treatment, although they probably do not represent the typical patient profile of Nephrology units due to the high prevalence of FH and patients intolerant to statins.
The main strength of this study is that it is a national study that describes the clinical profile and therapeutic management of the first patients with hyperlipidemia treated with evolocumab, which provides information of interest on the clinical benefit of this drug in CKD patients. The broad spectrum of indications has made it possible to observe that the drug is effective in different stages of CKD and, although the number of patients with CKD stage 3 and 4 was small (n= 8), we observed a similar reduction in the figures for c-LDL in these patients compared to patients with a lower degree of CKD. Another very relevant aspect was the efficacy demonstrated in patients with nephrotic syndrome, since in some of these patients the control of lipid alterations with standard therapy is very difficult.
These results suggest that the use of PCSK9 inhibitors as therapy added to statins could allow an additional reduction of LDL-C in patients with CKD with and without nephrotic syndrome, allowing us to achieve the therapeutic objectives indicated by the current guidelines. The use of therapeutic targets for reducing LDL-C levels based on the patients' baseline CV risk and the final LDL-C target should be the strategy for considering treatment with high-potency statins, with or without ezetimibe and inhibitors of PCSK9.
FundingThis study was funded by Amgen through an unrestricted grant.
Conflict of interestsM. Goicoechea has received fees for lectures from Amgen and as coordinator of the RETOSS NEFRO study; G. Martín-Reyes has received fees for conferences and travel grants for attendance at conferences and educational meetings from Amgen; S. Bea declares having given lectures for Boehringer, Lilly, Novonordisk, Astra, Esteve; J. de Juan-Ribera has received travel grants to attend conferences and educational meetings from Amgen; JL Górriz has received honoraria from Amgen to deliver lectures; S. Villamayor is employed by Amgen; The rest of the authors declare that they have no conflict of interest.
Amgen’s medical department designed the study in collaboration with the national study coordinator and principal investigators from each center (Scientific Committee).
The manuscript has been prepared by Montse Sabaté from TFS SL and reviewed by the scientific coordinator of the study and validated by the scientific committee.
Please cite this article as: Goicoechea M, Álvarez V, Segarra A, Polaina M, Martín-Reyes G, Robles NR, et al. Perfil clínico de los pacientes tratados con evolocumab en unidades hospitalarias de nefrología en España (RETOSS-NEFRO). Nefrologia. 2022;42:301–310.