Remdesivir is the only antiviral treatment that has been shown to be useful against SARS-CoV-2 infection. It shorts hospitalization time compared to placebo. Its effects in Kidney transplant (KT) patients are limited to some published cases.
MethodsWe performed a retrospective observational study that included all KT patients admitted between August 01, 2020 and December 31, 2020 with SARS-CoV-2 pneumonia who received remdesivir.
The objective of this study was to describe the experience of a cohort of KT patients treated with remdesivir.
DiscussionA total of 37 KT patients developed SARS-CoV-2 infection, 7 of them received treatment with remdesivir. The rest of the patients did not receive the drug due to either CKD-EPI less than 30 mL/min or they did not present clinical criteria. In addition to remdesivir, all pacients received dexamethasone and anticoagulation therapy. 4 were men, the median age was 59 (53–71) years. Median time from transplantation was 43 (16–82) months. Chest X-rays of all patients showed pulmonary infiltrates and required low oxygen flow therapy upon admission, requiring high flow nasal therapy in 3 cases. Only 2 cases presented deterioration of the graft function, not requiring hemodialysis in any case, and all recovered renal function at hospital discharge. 2 patients rise up 1.5 times the liver function test. No patient died or required admission to the critical care unit. Median days of admission was 12 (9–27) days.
ConclusionsOur study suggests that the use of remdesivir could be useful in KT patients with SARS-CoV-2 pneumonia without side effects. Additional studies are necessary with a larger number of patients to improve the knowledge of this drug in SARS-CoV-2 infection.
El remdesivir es el único tratamiento antiviral que ha demostrado ser útil frente al SARS-CoV-2 acortando el tiempo de hospitalización frente a placebo. Su efecto en pacientes trasplantados renales (TR) se limita a algunos casos publicados.
Material y métodosEstudio retrospectivo observacional de los pacientes TR que ingresaron entre 01/08/2020 hasta el 31/12/2020 con neumonía por SARS-CoV-2 y recibieron remdesivir.
El objetivo es describir la experiencia de una cohorte de pacientes TR con neumonía por SARS-CoV-2 tratados con remdesivir.
Resultados37 pacientes TR ingresaron por infección secundaria a SARS-CoV-2, 7 de ellos recibieron tratamiento con remdesivir. El resto de pacientes fueron excluidos por CKD-EPI menor a 30 mL/min o por no presentar criterios clínicos. Además de remdesivir, todos recibieron dexametasona y anticoagulación. 4 eran hombres, siendo la mediana de edad de 59 (53–71) años. La mediana de tiempo post-trasplante fue de 43 (16–82) meses. Todos los pacientes presentaban neumonía y en 3 de ellos se precisó oxigenoterapia de alto flujo. 2 presentaron deterioro de la función del injerto al diagnóstico, no precisando en ningún caso hemodiálisis, y recuperándose al alta. 2 pacientes elevaron 1.5 veces el valor normal de las transaminasas. Ningún paciente falleció ni precisó ingreso en unidad de críticos. La mediana de días de ingreso fue de 12 (9–27) días.
ConclusionesNuestro estudio sugiere que el uso de remdesivir podría ser útil en los pacientes TR con neumonía por SARS-CoV-2 sin efectos secundarios. Son necesarios más estudios con un mayor número de pacientes para ampliar el conocimiento de este fármaco en la infección por SARS-CoV-2.
The coronavirus is a human and animal pathogen. At the end of 2019, a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified as a cause of pneumonia in a group of patients in Wuhan, a city in Hubei province (China).1 The SARS-CoV-2 virus, previously known as 2019-nCoV, is the agent responsible for coronavirus disease 2019 (COVID-19).2 The pathogenesis of respiratory damage in COVID-19 is similar to severe acute respiratory syndromes in its clinical spectrum and epidemiology.1–3 It can manifest as an asymptomatic or symptomatic infection, with a spectrum ranging from mild to severe pneumonia. Infection can be divided into three phases: mild infection, the pulmonary phase and the inflammatory phase.4 Given the spread of the disease, as well as the current epidemiological situation, various treatments for the infection are being studied. Remdesivir is the only antiviral drug that has been shown to be useful against SARS-CoV-2 in the pulmonary phase, shortening hospitalisation time as compared to placebo.3
Kidney transplant (KT) patients are especially vulnerable to this disease, not just because they are immunosuppressed, but also because they are patients with different degrees of renal insufficiency. In the aforementioned studies assessing the efficacy of remdesivir, KT patients were not included. The effect in this population being limited to some published cases.5 In an observational study that included 46 patients with SARS-CoV-2 pneumonia with chronic kidney failure (16), kidney transplant recipients (8) and with acute kidney failure (22), the median number of days from admission to start of remdesivir was five (1–26) days, with 36 patients (78.2%) on dialysis at the start of remdesivir therapy. Of these, treatment was not completed in six patients due to death following clinical progression of the disease, and treatment was stopped in two patients due to clinical improvement. A single patient manifested a reaction to the infusion; transient behavioural changes were observed in five cases and acute gout was observed in one patient while undergoing treatment. In this study, good tolerance to remdesivir was observed in patients with chronic kidney disease, even in patients on dialysis.6
There are no reported cases of interaction between remdesivir and immunosuppressive therapy. However, given the limited experience in the field of interaction of both treatments, it is advisable to exercise caution and closely monitor the concentration of immunosuppression when administering with remdesivir due to the lack of knowledge and safety assessment studies.7
Given the limited evidence on the use of remdesivir in KT patients, it would be necessary to include this vulnerable group of patients in randomised clinical trials, but in the meanwhile, it is essential to describe the effect of remdesivir in this population.
The objective of our study was to retrospectively describe the clinical course of KT patients with SARS-CoV-2 infection who received treatment with remdesivir.
Materials and methodsStudy population and designA retrospective descriptive observational study was conducted that included all KT adults who were being followed up at the Hospital Universitario Germans Trias i Pujol [Germans Trias i Pujol University Hospital], who were diagnosed with COVID-19 between 1 August 2020 and 31 December 2020 and who received treatment with remdesivir. The clinical characteristics of the patients, laboratory data, treatments received, management of immunosuppression, graft function and clinical course were collected, extracting these data from the patients' clinical histories. Given the descriptive nature of the study, informed consent was not requested. The study was approved by the Hospital Ethics Committee.
TreatmentAntiviral treatmentRemdesivir is an antiviral nucleotide analogue that blocks ribonucleic acid (RNA) polymerase. A clinical trial conducted in China showed no statistically significant difference in time to clinical improvement between remdesivir and placebo.8 In contrast, the results of an international clinical trial revealed a shorter recovery time with remdesivir compared to placebo (10 days vs 15 days).3 The medical indication currently authorised in Spain is in hospitalised patients with pneumonia secondary to SARS-CoV-2 with a maximum of seven days from the onset of symptoms, who require low-flow supplemental oxygen and who meet two of the following criteria: respiratory rate (RR) greater than 24 rpm, O2 saturation (O2 Sat) less than 94% on room air and ratio of partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2 /FiO2) less than 300 mmHg. Remdesivir is contraindicated in patients with alanine aminotransferase (ALT) above five times the upper limit of normal and in patients with a glomerular filtration rate measured by CKD-EPI less than 30 ml/min/1.73 m2. Its use is not recommended in patients who require high-flow oxygen therapy, mechanical ventilation (invasive or noninvasive), extracorporeal membrane oxygenation (ECMO), or in patients who require vasoactive drugs to maintain blood pressure, in pregnant or breast-feeding women, or in patients with evidence of multi-organ failure. Following the recommendations of the Spanish National Transplant Organisation (ONT), the same protocol was established that is applied to the general population.9 The patients were administered a loading dose of 200 mg intravenously on the first day, followed by a maintenance dose of 100 mg intravenously per day, for five days. The protocol used at our hospital followed the recommendations for pharmacological treatment of adult patients with SARS-CoV-2 infection in the setting of the Integral Public Health System Utility of Catalonia (SISCAT).10
Corticosteroid treatmentPatients with COVID-19 who had had symptoms for more than seven days and with O2Sat under 94% in room air who needed additional oxygen, mechanical ventilation or ECMO11 were candidates for treatment with dexamethasone at a dose of 6 mg/24 h orally or intravenously for 10 days.
Anticoagulant therapyAll patients with COVID-19 received treatment with prophylactic enoxaparin at a dose of 1 mg/kg/day in patients with CKD-EPI greater than 30 ml/min/1.73 m2, and 0.5 mg/kg/day in patients with CKD-EPI less than 30 ml/min/1.73 m2.
Statistical analysisThe results are shown in the qualitative variables as numbers (fractions) and in the quantitative variables as medians (interquartile range).
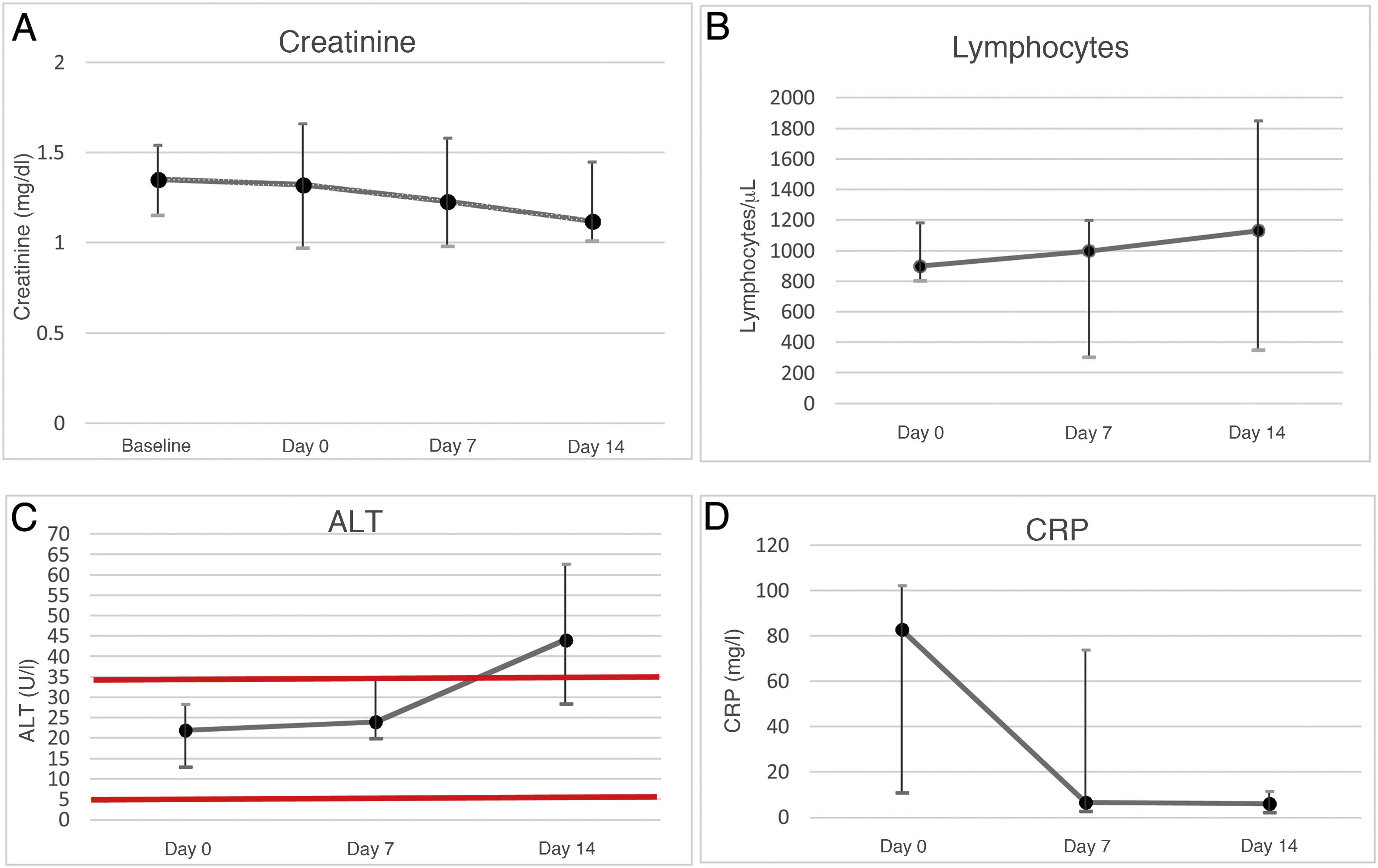
ResultsOf a total of 37 KT patients admitted with SARS-CoV-2 pneumonia, seven received treatment with remdesivir with a median of six (3–9) days elapsed since the onset of symptoms. The rest of the patients were excluded because of a CKD-EPI less than 30 ml/min or because they did not meet the clinical criteria. The median age was 59 (53–71) years. Demographic characteristics and the immunosuppression patients received are given in Table 1. The median post-transplant time was 43 (16–82) months, with the median renal function at admission being CKD-EPI 47 (40–75) ml/min/1.73 m2. The most common clinical manifestation was dyspnoea (6/7), followed by dry cough (5/7), tachypnoea (5/7) and fever (4/7). All patients showed bilateral infiltrates in the chest X ray and required low-flow oxygen therapy on admission with a baseline PaO2/FiO2 of 325 (206–373), with three patients requiring high-flow oxygen therapy throughout admission. In all patients, immunosuppressive therapy was suspended on the day of admission, only maintaining dexamethasone 6 mg. The median time to reintroduction of tacrolimus was 10 (8−18) days. Mycophenolic acid (MPA) was started in only two patients two months after discharge. Table 2 shows the laboratory characteristics of the patients. Two patients presented with impaired graft function due to acute tubular necrosis of multifactorial origin secondary to hypovolaemia, nephrotoxic agents and SARS-CoV-2 infection at the time of admission, prior to starting remdesivir, with peak creatinine of 2.2 mg/dl on the day of admission in the first case, and peak creatinine of 2 mg/dl in a recently transplanted patient, neither case requiring haemodialysis and both cases recovered normal kidney function on the 10th day of admission (Fig. 1A). The degree of lymphocytopenia mas maximal on admission, subsequently it recovered progressively (Fig. 1B). ALT increased progressively, it being considered a mild adverse effect secondary to treatment with remdesivir. It did not reach levels that double the normal limit in any case (Fig. 1C), while CRP decreased progressively during admission (Fig. 1D). No patient died, required admission to the intensive care unit or suffered other side effects to the treatment. The median length of hospital stay was 12 (10–18) days.
Medical history and treatment.
| Medical history (n = 7) | |
|---|---|
| Gender | Male: 4 |
| Female: 3 | |
| HTN | 6 |
| DLP | 5 |
| DM | 2 |
| BMI >30 | 2 |
| Pulmonary disease | 2 |
| - 1: SAHS | |
| - 1: past TB | |
| VitD <20 (ng/mL) | 2 |
| CKD | Not determined: 4 |
| ADPKD: 3 | |
| Kidney transplant | DBD cadaver: 7 |
| Thymoglobulin induction: 3 |
| Treatment (n = 7) | |
|---|---|
| Antibiotic | 4 |
| Remdesivir | 7 |
| Dexamethasone | 7 |
| Anticoagulation | 7 |
| Tacrolimus | Taken: 7 |
| Withdrawn: 7 | |
| Re-introduction: 10 (8−18) days | |
| Mycophenolic acid | Taken: 6 |
| Withdrawn: 6 | |
| Reintroduction: 4 not reintroduced; in 2 patients at median 55 days. | |
| mTORi | Taken: 0 |
| ACE inhibitors | Taken: 2 |
| Withdrawn during admission: 1 | |
| Reintroduced on discharge: 1 |
ACE inhibitor: angiotensin converting enzyme inhibitors; BMI: body mass index; CKD: chronic kidney disease; DLP: dyslipidaemia; DM: diabetes mellitus; HTN: hypertension; MTORi: mammalian target of rapamycin inhibitors; VitD: vitamin D.
Inflammatory biomarkers in kidney transplant patients with COVID-19 pneumonia.
| Normal range | Day 0 | Day 7 | Day 14 | |
|---|---|---|---|---|
| Leukocytes /μl | 4,000−11,000 | 6,300 (6,000−12,100) | 7,000 (5,400−9,200) | 9,225 (6,950−11,775) |
| Neutrophils /μl | 1,500−6,500 | 4,600 (4,000−10,500) | 5,800 (3,700−6,500) | 7,400 (5,650−9,265.75) |
| Lymphocytes /μl | 1,200−3,500 | 900 (800−1,185) | 1,000 (300−1,200) | 1,131.50 (350−1,850) |
| Haemoglobin g/dl | 12−16 | 13 (12.60−14) | 12.70 (11.20−14.10) | 13.20 (10.70−14.33) |
| Platelets ×103/μl | 150−400 | 178 (122−233) | 270 (181−295) | 187.5 (145.75−249.50) |
| ALT U/L | 5−35 | 22 (13−28.50) | 24 (20−35) | 44.10 (28.50−62.75) |
| D-dimer μg/mL | 0−500 | 1,047 (719.5−1,410) | 835.5** | 4,204.50** |
| LDH U/L | 135−247 | 253 (175.25−353.75) | 208 (189−335) | 220.50** |
| CK U/L | 0−145 | 75 (36.75−138.25) | 13* | |
| IL-6 pg/dL | 0−6.4 | 25.76 (10.37−102.25) | 88.08* | |
| Ferritin ng/dL | 30−400 | 551 (358.5−1,198.2) | 835 (378−1,211.10) | |
| Procalcitonin ng/mL | 0 | 0.10 (0.03−0.23) | 0.1* | 0.06* |
| CRP mg/L | 0−5 | 82.80 (10.60−102.25) | 6.60 (2.60−73.90) | 6 (1.93−11.50) |
| Creatinine mg/dL | 0.6−1.2 | 1.32 (0.97−1.66) | 1.23 (0.98−1.58) | 1.12 (1.01−1.45) |
ALT, alanine aminotransferase; CK, creatine kinase; IL-6, interleukin-6; LDH, lactate dehydrogenase; PCR, polymerase chain reaction.
A. Evolution of creatinine in the laboratory tests of kidney transplant patients (n = 7) based on baseline creatinine and its evolution during admission (day 0, day +7 and day +14). The creatinine median and interquartile range show kidney function stability during admission.
B. Evolution of lymphocytes in the laboratory tests of kidney transplant patients (n = 7) from admission (day 0, day +7 and day +14). The lymphocytes median and interquartile range show a progressive increase from admission, with the start of recovery without reaching normality on day 14.
C. Evolution of the ALT enzyme in the laboratory tests of kidney transplant patients (n = 7) from admission (day 0, day +7 and day +14). The ALT median and interquartile range show a progressive increase during admission. The horizontal red lines show normal values.
D. Evolution of CRP in the laboratory tests of kidney transplant patients (n = 7) from admission (day 0, day +7 and day +14). The CRP median and interquartile range show a decrease during the first seven days of admission.
There is currently no known treatment for COVID-19, so different treatment lines have been tried, including remdesivir.3 This study describes the clinical course of a group of KT patients with COVID-19 treated with remdesivir.
Remdesivir is a nucleoside analogue with antiviral activity. As a ribonucleic acid (RNA) polymerase inhibitor, it can inhibit the replication of multiple coronaviruses in respiratory epithelial cells. In monkey models infected with Middle East respiratory syndrome (MERS-CoV), treatment with remdesivir 24 h prior to infection achieved complete prevention of symptoms by inhibiting viral replication in the respiratory tract and preventing the formation of lung lesions. By administering remdesivir 12 h after infection, clear clinical benefits were observed, with reduced symptoms, virus replication in the lungs and pulmonary lesions.12 In human studies, remdesivir resulted in a shorter hospital stay compared to placebo when administered on day nine (7–12) of infection.3 In our study, it was started a median of six (3–9) days after the onset of symptoms. Therefore, it would be expected that the shorter the progression of the disease should reflect a more effective the treatment with remdesivir.
One study focused on the detection of side effects. The study included eight KT patients and found no serious adverse effects.6 We have not found other studies that assess the efficacy of remdesivir on KT patients with COVID-19.
Regarding the management of immunosuppression, it is a complex issue in the context of a severe infection, not only because of the infection itself, but also because of the possible interaction with the drugs used to treat SARS-CoV-2 pneumonia. It is important to mention that, to date, remdesivir has shown no interactions with calcineurin inhibitors, antimetabolites or mTORi.13,14 However, remdesivir can induce cytochrome p450 enzymes, including CYP1A2, CYP2B6 and CYP3A4.13,14 In our study, immunosuppression with calcineurin inhibitors and antimetabolites was suspended in all patients at diagnosis prior to starting remdesivir. None of the patients had previously suffered from rejection and therefore, to assess the benefit against the potential risk, only treatment with steroids was maintained.
The main side effects of remdesivir are hepatotoxicity and nephrotoxicity.3 Remdesivir is currently contraindicated in patients with ALT five times the upper limit of normal and in patients with a glomerular filtration rate less than 30 ml/min/1.73 m2. Beigel et al. published a cohort of 155 patients with SARS-CoV-2 infection treated with remdesivir, in which only 1% had to discontinue treatment due to acute kidney failure.3 Natasha et al. did not find a higher incidence of adverse effects, impaired kidney function or elevated transaminases among patients with a glomerular filtration rate higher or lower than 30 ml/min/1.73 m2.15 In our study, there were only two cases of impaired kidney function prior to the administration of remdesivir. Regarding hepatotoxicity, there was a slow but progressive increase in ALT, but transaminase values barely doubled normal levels.
Remdesivir is the only antiviral therapy that has been shown to be useful against SARS-CoV-2 in the pulmonary phase, and it shows a shortening of recovery time compared to placebo (median 10 days vs 15 days)3 and shortening hospital stay in patients who received this treatment versus placebo (median 12 days versus 17 days).3 In a review that included 420 cases from the first wave, where no patient received treatment with remdesivir, the length of hospitalisation was 16 (1–100) days.16 During the same period in Spain, the duration of hospital stay was 14 (8–21) days.17 In our study, the median days of admission and recovery in KT patients with SARS-CoV-2 pneumonia was 12 (10–18) days, suggesting a decrease in the length of hospital stay in KT patients when they receive treatment with remdesivir.
The results of different studies show a mortality rate of 28% at three weeks in KT patients compared to 1%–5% of the general population prior to the approval of remdesivir.18 In the study by Villanego et al., of the 1,011 cases included, 548 were from the first wave and 463 from the second (93.6% and 80.4%, respectively, of the total number of patients included in the registry). In the second wave, KT patients were younger, 18.4% were asymptomatic and had fewer cases of pneumonia (49.7% vs 80.5%). During the first wave, there were changes in treatment with increased use of remdesivir and steroids. Ritonavir/lopinavir, hydroxychloroquine and azithromycin were hardly used in the second wave. Hospitalisation decreased (63.3% in the second wave compared to 90% in the first), but more KT recipients were admitted to the ICU, when patients hospitalised during the second wave were analysed. Overall mortality in this cohort was lower (even excluding asymptomatic cases) during the second wave (15.1%) compared to the first wave (27.4%) (p < 0.001). Mortality of hospitalised patients was also lower (22.9% in the second wave compared to 29.5% in the first wave, p < 0.04). However, in critical KT patients, mortality was 66.7%, not significantly different from that reported in the first wave (58.1%).17 In our series of patients treated with remdesivir for five days, clinical course was favourable without need of admission to the intensive care unit, and patients were discharged with a mortality rate of 0%, which suggests that remdesivir could be an effective treatment.
Regarding prognosis, Beigel et al. suggest that treatment with remdesivir in the general population may have prevented progression to more severe respiratory disease, and it may have decreased the use of the scarce healthcare resources during the pandemic.3 In our study, all patients showed with bilateral infiltrates on x-ray scans and required low-flow oxygen therapy at diagnosis. After starting treatment with remdesivir, only three patients required high-flow oxygen therapy, without admission to the intensive care unit or invasive mechanical ventilation being required in any of the cases.
With respect to the incidence of acute kidney failure, the aforementioned review, which includes kidney transplant recipients from the first wave, reports an incidence of 44%, compared to 29% in the general population.16 A substantial proportion of those who developed kidney failure (23%) required renal replacement therapy. Of the remaining patients, most cases recovered kidney function at discharge.16 In our study of patients treated with remdesivir, there were two cases of impaired kidney function that worsened 1.5 times the baseline value at diagnosis. The cause of impaired kidney function was attributed to acute tubular necrosis secondary to hypovolaemia, nephrotoxic agents and SARS-CoV-2 infection (no kidney biopsy performed in any case). It was not attributed to a side effect to remdesivir in any of the cases, with progressive recovery during admission, reaching baseline creatinine on the 10th day of admission.
On one hand, our study is one of the first published including cohorts of KT with COVID-19 pneumonia treated with remdesivir, analysing its efficacy and safety. On the other hand, it is a retrospective observational study with a small sample size with its consequent biases.
ConclusionsOur study suggests that KT patients with SARS-CoV-2 infection undergoing treatment with remdesivir show a good clinical course with no adverse effects and low mortality. Remdesivir is well tolerated in kidney transplant patients, with no evidence of nephrotoxicity related to the drug. More controlled studies with a larger number of patients are needed to broaden our understanding the effect of this drug on SARS-CoV-2 infection in kidney transplant patients.
- 1
Remdesivir is the only antiviral drug that has been shown to be useful against SARS-CoV-2, shortening hospitalisation time compared to placebo.
- 2
In animal studies, remdesivir demonstrates better antiviral effect when the drug is administered earlier.
- 3
The main side effects of remdesivir are nephrotoxicity and hepatotoxicity. However, there are observational studies that suggest good tolerance to remdesivir in patients with CKD, including dialysis and transplant patients.
- 4
Remdesivir in kidney transplant patients has been administered without adverse effects.
- 5
No interactions between remdesivir and immunosuppressive therapy have been described. However, given the limited experience, it is recommended to actively monitor the immunosuppressant concentration when they are administered concomitantly.
- 6
KT patients with SARS-CoV-2 infection undergoing treatment with remdesivir show a good clinical course with no adverse effects and low mortality.
None.
Conflicts of interestThe authors have no conflicts of interest.