Percutaneous left atrial appendage closure (LAAC) has been proposed as an alternative to anticoagulation therapy in patients with nonvalvular atrial fibrillation (NVAF) to decrease the thromboembolic risk, while avoiding the risks of chronic anticoagulation. This option may be attractive in patients with NVAF and chronic kidney disease (CKD), since they exhibit both high thromboembolic and bleeding risks.
ObjectiveTo evaluate the prognostic impact of the presence of CKD in patients with atrial fibrillation undergoing LAAC peri-procedure and during the follow-up as compared with patients with preserved renal function.
MethodsRetrospective, observational study that included 124 consecutive patients with atrial fibrillation undergoing LAAC in a university hospital, and the results were evaluated according to the baseline renal function of the patients.
ResultsThe median age was 75,5 years (IQR 67,6–80) and 62,1% were men, the median of CHA2DS2-Vasc and HASBLED scores was 4 (IQR 3−4) for both scores. Up to 57,3% of the total sample had CKD. Baseline characteristics were similar between groups, but CKD patients were older and had a higher HASBLED score. During the procedure, no thromboembolic, bleeding events, or deaths were observed. Combining the time of hospitalization and follow-up, no significant differences were observed between groups in the annual rate of thromboembolic events (0.97/100 patient-years [100PY] vs 4.06/100PY, P =,09), but there was a higher rate of bleeding events (5.67/100PY vs. 13.3/100PY, P =,033) and mortality among CKD patients (6.50/100PY vs. 17.2/100PY, P =,009), with an odds ratio of 2.711 (95% CI 1,96–6,95). In the multivariate analysis a preserved eGFR was independently associated with a lower mortality risk.
ConclusionsLAAC is a valid alternative to oral anticoagulation in patients with CKD and atrial fibrillation, with a low rate of peri- and post-procedure complications, although CKD patients exhibited a higher risk of bleeding and mortality during the follow-up. However, these higher rates may not be necessarily related to the procedure.
El cierre percutáneo de la orejuela izquierda (CPOI) se ha propuesto como una alternativa al tratamiento anticoagulante en pacientes con fibrilación auricular no valvular (FANV) para disminuir el riesgo tromboembólico, evitando los riesgos de la anticoagulación. Esta opción puede ser atractiva en pacientes con FANV y enfermedad renal crónica (ERC), ya que presentan un alto riesgo tanto tromboembólico como hemorrágico.
ObjetivoDeterminar el papel pronóstico de la presencia de ERC en pacientes con fibrilación auricular sometidos a un CPOI peri-procedimiento y durante el seguimiento comparado con los pacientes con función renal preservada.
MétodosEstudio retrospectivo, observacional de muestreo consecutivo que incluyó 124 pacientes sometidos a CPOI por fibrilación auricular en un hospital universitario y se compararon los resultados según la función renal basal.
ResultadosLa mediana de edad fue 75,5 años (RIQ 67,6–80), el 62,1% eran hombres, la mediana de CHA2DS2-Vasc y HASBLED era de 4 (RIQ 3−4) para ambas escalas. El 57,3% de la muestra tenía ERC. Las características basales eran similares entre ambos grupos, salvo una media de edad y un score de HASBLED superiores en los pacientes con ERC. Durante el procedimiento, no se observó ningún evento tromboembólico, de sangrado o muerte. Durante el tiempo de hospitalización y de seguimiento combinados, no hubo diferencias significativas entre grupos en la tasa anual de episodios tromboembólicos (0,97/100 pacientes-año [100PA] vs 4,06/100PA, P =,092), pero si se observó una mayor tasa de sangrados (5,67/100PA vs 13,3/100PA, P =,033), y de mortalidad (6,50/100PA vs 17,2/100PA, P =,009) en el grupo de ERC, con una odds ratio para mortalidad de 2,711 (IC95% 1,96–6,95). En el análisis multivariante, el FGe preservado se asoció independientemente con una menor mortalidad.
ConclusionesEl CPOI es una alternativa válida a la anticoagulación oral en pacientes con ERC y fibrilación auricular, con una baja tasa de eventos peri y post-procedimiento. No obstante, los pacientes con ERC presentan mayor riesgo de sangrado y de mortalidad durante el seguimiento. Aunque estas mayores tasas no están necesariamente relacionadas con el procedimiento.
Non-valvular atrial fibrillation (NVAF) is the most common chronic arrhythmia in the world1 and it is associated with increased morbidity and mortality due to the thromboembolic risk it entails. For this reason, the guidelines recommend thromboprophylaxis with oral anticoagulation (OAC), either anti-vitamin K agents (AVK or coumarin agents) and, more recently, direct-acting oral anticoagulants (DOAC), in patients with a high thrombotic risk, as generally evaluated by CHA2 DS2-Vasc score.2
Patients with chronic kidney disease (CKD) have a higher incidence of NVAF than the general population,3 and its prevalence increases with the deterioration of renal function. In addition, patients with CKD have a higher risk of thromboembolic events, as well as bleeding, which complicates their management. Additionally, advanced CKD patients treated with AVK remain in the INR therapeutic range for less time than patients with moderate CKD or without CKD, and this is associated to an increased risk of thromboembolic events, bleeding, and mortality.5 In addition, AVK agents, such as acenocoumarol, favor vascular calcification, since they inhibit the γ -carboxylation vitamin K dependent required by Matrix Gla Protein for its activation, a protein that inhibits vascular calcification.6 This may aggravate the increased cardiovascular risk and of vascular calcification associated with bone-mineral diseae, common in patients with CKD, as well as the risk of calciphylaxis.7
There is no evidence about safety and efficacy of OAC in CKD stage 4 or 5 patients since these patients have generally been excluded from the main randomized studies. Current AF management guidelines advise the use of DOACs versus VKA for its best risk-benefit profile in patients with preserved kidney function or with CKD stage 3. In CKD stage 4 and, especially, stage 5 or patients on dialysis, the guidelines make non uniform recommendations given the lack of evidence on its efficacy and safety of both VKAs and DOACs, as mentioned already.9–12 All of the above makes it difficult to decide whether or not to start OACs in advanced stages of CKD.
Percutaneous left atrial appendage closure (LAAC) is a non-pharmacological alternative to oral anticoagulation in patients with NVAF, aiming to reduce thromboembolic risk.13,14 In sinus rhythm, the left atrial appendage (LAA) is a contractile structure that empties its contents into the ventricle with each beat. In contrast, in NVAF, the LAA loses its contractile capacity and progressively dilates, favoring blood stasis and consequently increasing the risk of intraluminal thrombosis. This is why, in patients with NVAF, 90% of thrombi in the left atrium are located or originate in the LAA.15,16 This is aggravated in the presence of CKD, which is a risk factor for the presence of thrombi in the LAA in patients with NVAF.17 Thus, the fact that patients with percutaneous LAAC do not require oral anticoagulation makes it an attractive option for CKD patients, especially patients with advanced CKD or on dialysis, for the reasons mentioned above. However, and given that in patients with CKD invasive procedures are associated with higher morbidity and mortality, studies in this population are necessary to confirm their efficacy and safety.18
The primary objective of this study was to determine whether patients with decreased renal function (defined as baseline estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2) treated with percutaneous LAAC had a higher incidence of thromboembolic events, bleeding and mortality than patients with preserved renal function. The secondary objectives were to assess the differences between the group with preserved and decreased renal function in terms of the procedure and their complications, and also evaluate the changes in renal function in both groups after the procedure.
Patients and methodsThis is an observational, retrospective study that has analyzed consecutive patients undergoing percutaneous LAAC performed at a university hospital between January 2011 and July 2019. All patients that underwent the procedure were included. The main inclusion criteria for this intervention were: patients with an indication for anticoagulation who for some reason could not use oral anticoagulants, or patients with a stroke of cardioembolic origin despite anticoagulant treatment. The main exclusion criteria for the technique were: contraindication for transesophageal catheter, thrombi protruding into the lumen of the appendage, active bleeding or recent stroke (less than three months).
We collected the main demographic data, baseline variables (comorbidities, anticoagulant/antiplatelet treatment), renal function at baseline and after the procedure, cause of contraindication for anticoagulation, characteristics and complications of the procedure, and complications during follow-up. The data was recorded in an anonymized database to protect the identity of the patients.
The renal function of the patients was evaluated by means of the estimated glomerular filtration rate (eGFR) at baseline and at discharge, using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.19 The patients were classified into the following renal function stages according to the criteria of the Kidney Disease Improving Global Outcomes (KDIGO) guidelines20: stage 3a (eGFR 45−59 mL/min/1.73 m2), stage 3b (eGFR 30–44 mL/min/1.73 m2), stage 4 (eGFR 15−29 mL/min/1.73 m2) and stage 5 (eGFR < 15 mL/min/1.73 m2 and/or dialysis).
The different stages of atrial fibrillation were defined according to the criteria of the European Society of Cardiology21: paroxysmal AF was defined as self-limited episodes of AF lasting less than 7 days. Persistent AF was defined as episodes lasting 7 days or longer that required electrical cardioversion to be reversed. Permanent AF was defined as AF lasting more than 7 days and in which the doctor and patient jointly decide not to consider restoring sinus rhythm, neither pharmacologically nor electrically. Thrombotic (CHA2 DS2 -Vasc) and hemorrhagic (HASBLED) risk assessment scores were calculated for all patients.2,22
Percutaneous LAAC closure is a procedure performed under deep sedation or general anesthesia and guided by transesophageal ultrasound (TEE). The first ultrasound examination allows the to analyze the morphology of the LAA and to ruled out the presence of thrombi. TEE can also assess the presence and degree of preprocedural pericardial effusion, mitral valve function, and left superior pulmonary vein patency. Subsequently, several baseline measurements of the LAA are obtained in different surface levels.
Vascular access is preferably through right femoral vein,since it facilitates transseptal puncture, which is performed in the posterior and lower part of the interatrial septum under fluoroscopic and TEE guidance. Then, the transseptal catheter is changed by the catheter used to deliver the device which is placed in the area of the LAA implantation (10−15 mm from the ostium). Finally, the position and stability of the device is confirmed by TEE. A successful procedure was defined as the correct implantation of the device in the LAA.23
After the procedure, the antithrombotic treatment usually prescribed to prevent the formation of thrombi on the device was dualantiplatelet therapy for three months, and subsequently, monotherapy with acetylsalicylic acid (ASA). However, in recent months, treatment with low-dose DOACs for three months has been proposed as an alternative.
The patients underwent routine clinical follow-up at the first, third and twelfth month after the procedure. During follow-up, a TEE was performed 3−6 months after the procedure. In some cases, and always following the usual clinical protocols, patients were contacted by telephone after twelve months. Data on mortality, thromboembolic events, and bleeding were collected. The presence of thrombi on the device documented by TEE was recorded.
Stroke, systemic embolism and transient ischemic attack (TIA) were considered Thromboembolic events. Bleeding episodes were classified according to the Bleeding Academic Research Consortium (BARC) definition: Type 0 is no evidence of bleeding; Type 1 corresponds to bleeding that does not require medical attention; Type 2 is a blood loss that requires diagnostic studies, hospitalization, or treatment by a health professional, and does not meet the criteria for types 3, 4, or 5; Type 3 is divided into three subtypes: 3a is bleeding with decrease in hemoglobin ≥3 and <5 g/dL or requiring transfusion, 3b is bleeding with a decrease in hemoglobin ≥5 g/dL or bleeding requiring surgery or intravenous vasoactive drugs, or cardiac tamponade; and 3c includes intracerebral hemorrhage and intraocular bleeding compromising vision. Type 4 includes bleeding related to coronary bypass surgery, and type 5 is fatal bleeding that could or does lead to death. Major bleeding was defined as ≥3a of the BARC classification.24
The study met all the ethical criteria of the institution where the study was carried out (Hospital Clínic de Barcelona). All patients signed the informed consent before the procedure.
Statistic analysisQualitative variables are expressed as absolute and relative frequencies, as well as annual rate (number × 100 patients/year), as appropriate, and quantitative variables are shown as as mean ± standard deviation or as median (interquartile range [IQR]) depending on whether the distribution was normal or not, according to the Kolmogorov–Smirnov test. Quantitative variables from two groups were compared using the Student's t-test for independent data if they followed a normal distribution, or the Mann–Whitney U test otherwise. The Chi-square test has been used to compare qualitative variables.
Survival was defined as the time elapsed from the date of the procedure to the last follow-up or death of the patient. Survival curves for the groups of interest were calculated according to the Kaplan–Meier method. The odds ratio was calculated to determine the relationship between the presence or absence of decreased renal function and post-procedure mortality. Since differences in baseline characteristics could substantially interfere with the results, a sensitivity analysis was performed using a Cox multivariate regression model.
The level of significance was considered for a P-value less than .05. All statistical analyzes were performed with the statistical program IBM SPSS Statistics version 25 for Windows.
ResultsDuring the study period, 124 consecutive patients with atrial fibrillation undergoing LAAC were included in the study (42.7% patients with preserved eGFR and 57.3% with reduced eGFR).
Table 1 shows the baseline characteristics of the patients, as well as the anticoagulant/antiplatelet treatment prescribed before the intervention in the preserved and reduced renal function subgroups. The median age of the patients was 75.5 (IQR 67.6−80) years and the predominant sex was male (62.1%). The median for CHA2 DS2 -Vasc was 4 (IQR 3−4) and for HASBLED 4 (IQR 3−4). No significant differences were observed between the preserved and reduced renal function groups in terms of baseline patient characteristics, except for age, which was higher in the reduced eGFR group (70.5 years [IQR 65−78] vs. 77.0 years [IQR 69−81], P = .048) and the risk of bleeding (HASBLED 3 [IQR 3−4] vs. 4 [IQR 3−4], P = .026). Eleven patients had previous heart valve repair surgery. Out of the 100 patients with a history of bleeding, 45% were gastrointestinal hemorrhages, 38% had intracranial hemorrhages, 6% bleed in the ENT area, 3% had respiratory bleeding, 1% abdominal bleeding, 1% had retroperitoneal hematoma and 6% from other origins (gluteal hematoma, 2 hematomas related to dialysis, ophthalmic bleeding, hematuria, and one bleeding related to infiltrations). Median baseline creatinine was 1.16 mg/dL (IQR 0.88–1.54); in the preserved renal function group the median baseline creatinine was 0.86 mg/dL (IQR 0.76−1) and 1.48 mg/dL (IQR 1.25−1.90) in the reduced renal function group (excluding those dialysis patients). Among the 71 patients with reduced eGFR, 51 were in stage 3 (71.8%), 10 in stage 4–5 non-D (14.1%) and 10 patients in stage 5 on dialysis (14.1%). All patients had an absolute or relative contraindication for OAC (96.8%) or a history of thromboembolic stroke despite anticoagulation (3.2%) (in Appendix B Table 1 of the supplementary material shows the characteristics according to CKD stage, significant differences in age, and CHA2 DS2 -Vasc and HASBLED scores are observed between the 3 groups).
Baseline characteristics of the patients.
| Total (n = 124) | eGFR> 60 (n = 53) | eGFR <60 (n = 71) | P | |
|---|---|---|---|---|
| Age, years | 75.5 (67.6−80) | 73 (65−78) | 77 (69−81) | .048 |
| Female sex, n (%) | 47 (37.90) | 21 (39.62) | 26 (36.62) | .733 |
| BMI (kg/m2) | 26.63 ± 4.36 | 27.28 ± 4.52 | 26.11 ± 4.18 | .178 |
| Arterial Hypertension, n (%) | 112 (90.32) | 47 (88.32) | 65 (91.55) | .593 |
| Diabetes mellitus, n (%) | 34 (27.42) | 11 (21.75) | 23 (32.39) | .151 |
| Atrial fibrillation, type | .409 | |||
| Paroxysmal, n (%) | 44 (37.29) | 22 (44.00) | 22 (32.35) | |
| Persistent, n (%) | 2 (1.70) | 1 (2.00) | 1 (1.47) | |
| Permanent, n (%) | 72 (61.02) | 27 (54.00) | 45 (66.18) | |
| History of stroke, n (%) | 42 (45.16) | 23 (56.10) | 19 (36.54) | .093 |
| History of TIA, n (%) | 7 (5.65) | 2 (3.77) | 5 (7.04) | .435 |
| History of systemic embolism, n (%) | 4 (3.23) | 2 (3.92) | 2 (2.82) | 1.000 |
| History of heart failure, n (%) | 40 (32.26) | 12 (24.53) | 27 (38.57) | .100 |
| History of vascular disease, n (%) | 29 (23.39) | 9 (16.98) | 20 (28.17) | .145 |
| Bleeding history, n (%) | 100 (80.65) | 42 (79.25) | 58 (81.69) | .733 |
| Left ventricular dysfunctiona, n (%) | 24 (19.35) | 7 (13.21) | 17 (24.29) | .125 |
| CHA 2 DS 2 Vasc Score | 4 (3−4) | 4 (3−5) | 4 (3−6) | .086 |
| HASBLE Score | 4 (3−4) | 3 (3−4) | 4 (3−4) | .026 |
| Baseline treatment | ||||
| ASA, n (%) | 40 (32.26) | 17 (33.33) | 23 (31.51) | .830 |
| ADP inhibitor, n (%) | 11 (8.87) | 5 (9.80) | 6 (8.22) | .486 |
| Warfarin, n (%) | 22 (17.74) | 11 (21.57) | 11 (15.07) | .351 |
| Others, n (%) | 33 (26.61) | 19 (33.00) | 14 (21,12) | .119 |
| Baseline creatinine, mg/dL b | 1.16 (0.88 1.54) | 0.86 (0.76−1) | 1.48 (1.25−1.9) | ≤.001 |
| Baseline estimated glomerular filtration rate c | 63.24 ± 33.01 | 86.32 ± 30.88 | 42.56 ± 17.59 | ≤.001 |
Mean ± standard deviation, median (interquartile range) or frequency (%).
ASA: acetylsalicylic acid; TIA: transient ischemic attack; BMI: body mass index.
Regarding the baseline anticoagulant/antiplatelet treatment, 10 patients (8.1%) received dual antiplatelet therapy, 2 patients received triple therapy (1.6%), 80 patients (64.5%) received a single drug, and 32 patients (25, 8%) received no treatment. Forty patients received ASA, 10 clopidogrel, 1 ticagrelor, 22 VKAs, and 33 were treated with other anticoagulants (mainly low molecular weight heparins and DOACs).
The TEE procedure was performed in 100% of the patients. In 12 cases (9.7%), a combined procedure was performed, mostly with percutaneous mitral valve repair with the MitraClip® device (Abbott Laboratories, Abbott Park Illinois, USA). The data related to the procedure can be seen in Table 2. Between the groups, there were no statistically significant differences in the ejection fraction of the left ventricle, nor in the amount of contrast administered, duration of fluoroscopy, or type of device implanted. The Amplatzer™ Cardiag Plug (ACP) and Amplatzer Amulet™ (St. Jude Medical, Minnesota, USA), Watchman™ (Boston Scientific, Massachusetts, USA) devices were used. USA) and others (the majority, LAmbre™, Lifetech Scientific; Shenzhen, China), the most frequent being the Amulet (70.16%). In Appendix B, Table 2 of the supplementary material, the data is presented according to the CKD stage, observing that patients with CKD stages 4–5 not on dialysis received on average less contrast during the procedure.
Procedure (features).
| Total | eGFR > 60 | eGFR < 60 | P | |
|---|---|---|---|---|
| LVEF | 60 (50−60) | 57 (50−60) | 60 (50−60) | .737 |
| Contrast, mL | 70 (50−108.5) | 70 (57.5−113) | 62.5 (45−103.5) | .487 |
| Fluoroscopy time, min | 13.85 (10−21.7) | 14.1 (11−20) | 13.7 (10−23) | .205 |
| Device type, n (%) | ||||
| ACP | 16 (12.90) | 5 (9.80) | 11 (15.07) | .783 |
| Amulet | 87 (70.16) | 38 (71.69) | 49 (69.01) | |
| Watchman | 2 (1.61) | 1 (1.96) | 1 (1.37) | |
| Others | 19 (15.32) | 9 (17.65) | 10 (13.70) |
Variables are expressed as median (interquartile range) or frequency (%).
ACP: Amplatzer Cardiac Plug; LVEF: left ventricular ejection fraction; eGFR: estimated glomerular filtration rate (mL/min/1.73 m2).
During the procedure there were complications in two cases: a pericardial effusion without cardiac tamponade and a pseudoaneurysm of the superficial femoral vein. No thromboembolic event, death, or device embolization was observed during the procedure. Correct implantation of the device in the LAA was achieved in 99.2% of cases, with no statistically significant differences between the group of patients with reduced eGFR and patients normal eGFR.
Regarding the anticoagulant/antiplatelet treatment of patients at discharge, 7 (5.65%) received no treatment, 58 (46.77%) received treatment with a single drug, and 59 patients (47.58%) received double antiplatelet treatment. Sixty-nine (55.6%) patients were treated with ASA, 63 (50.8%) patients with ADP inhibitors (62 with clopidogrel and 1 with ticagrelor), 2 (1.6%) patients treated with VKAs, 10 (8.1%) with enoxaparin and 32 (25.8%) treated with other anticoagulants (mainly DOACs). Treatment differences between patients with preserved and reduced renal function groups were not significant, except for ADP inhibitors, which were prescribed more frequently at discharge in the reduced eGFR group.
Follow-upThe results broken down by hospitalization and follow-up are shown in Table 3 (and in Appendix B Table 3 of the supplementary material according to CKD stage). Patients were followed up for a median of 567 (IQR 207−944) days. This represents a follow-up of 235.4 patient-years for the entire series (127.7 patient-years in the group of patients with decreased renal function and 107.7 patient-years in the group with preserved renal function).
Thromboembolic events, bleeding episodes and mortality during hospitalization and follow-up.
| Total | eGFR > 60 | eGFR < 60 | P | |
|---|---|---|---|---|
| Follow-up time (days) | 567 (207−944) | 567 (208–1021) | 592 (207–917) | .500 |
| Thromboembolic events, n (%)/and annual rate | ||||
| Hospitalization | 0 | 0 | 0 | NA |
| Follow-up | 6 (4.84)/2.66 | 1 (1.96)/0.97 | 5 (6.85)/4.06 | .092 |
| H + FU | 6 (4.84)/2.66 | 1 (1.96)/0.97 | 5 (6.85)/4.06 | .092 |
| Bleeding episodes, n (%)/and annual rate | ||||
| Hospitalization | 2 (1.61) | 0 | 2 (2.82) | .507 |
| Follow-up | 21 (16.94)/8.97 | 6 (11.76)/5.67 | 15 (20.55)/11.7 | .065 |
| H + FU | 23 (18.55)/9.82 | 6 (11.76)/5.67 | 17 (23.29)/13.3 | .033 |
| Mortality, n (%)/and annual rate | ||||
| Hospitalization | 1 (0.81) | 0 | 1 (1.63) | .401 |
| Follow-up | 28 (22.58)/12.1 | 7 (13.73)/6.50 | 21 (28.77)/17.1 | .009 |
| H + FU | 29 (23.39)/12.3 | 7 (13.73)/6.50 | 22 (30.14)/17.2 | .009 |
Variables expressed as frequency (%) and annual rate (in number per 100 patients/year) in follow-up and H + FU (Hospitalization plus Follow up).
eGFR: estimated glomerular filtration rate (mL/min/1.73 m2).
During hospitalization, one patient died (with reduced eGFR), two bleeding episodes were observed, both in the reduced eGFR group (2.74%), and there was no evidence of thromboembolic events. There were 3 significant pericardial effusions without cardiac tamponade, none of them required pericardiocentesis or surgery.
During follow-up, 28 patients died (7 patients in the preserved eGFR group [13.73%] and 21 patients in the reduced eGFR group [28.8%]). Twenty-one bleeding episodes were observed (6 patients in the preserved eGFR group [11.76%)] and 15 patients in the reduced eGFR group [20.55%]). The bleeding episodes during follow-up were: type 1 (n = 3), type 2 (n = 2), type 3a (n = 11), type 3b (n = 1), type 3c (n = 3) and of type 5 (n = 1). Of the major bleedings (BARC ≥ 3a), 3 were observed in patients with preserved eGFR and 13 in patients with decreased eGFR (P = .094). Of these bleeding episodes, 10 out of the 21 occurred during the first three months of antiplatelet/anticoagulant treatment. Likewise, 6 thromboembolic events were recorded (1 patient in the preserved eGFR group [1.96%] and 5 patients in the reduced eGFR group [6.85%]), (5 ischemic strokes and one transient ischemic attack) (Table 4).
Thromboembolic events, bleeding episodes and mortality observed during hospitalization and follow-up.
| Event | Total (n = 124) | eGFR > 60 mL/min/1.73 m2 (n = 53) | eGFR < 60 mL/min/1.73 m2 (n = 71) |
|---|---|---|---|
| Thromboembolic events, n (%) | |||
| Stroke | 5 (4.1) | 1 (1.9) | 4 (5.6) |
| TIA | 1 (0.8) | 0 | 1 (1.4) |
| Systemic embolism | 0 | 0 | 0 |
| Bleeding episodes, n (%) | |||
| Gastrointestinal | 10 (8.1) | 1 (1.9) | 9 (12.7) |
| Intracranial | 3 (2.4) | 1 (1.9) | 2 (2.8) |
| Others a | 10 (8.1) | 4 (7.5) | 6 (8.5) |
| Mortality, n (%) | |||
| Stroke | 2 (1.6) | 0 | 2 (2.8) |
| Heart failure | 3 (2.4) | 1 (1.9) | 2 (2.8) |
| Sudden death | 9 (7.6) | 4 (7.5) | 5 (7.1) |
| Infectious | 9 (7.6) | 1 (1.9) | 8 (11.3) |
| Bleeding | 3 (2.4) | 0 | 3 (4.2) |
| Others b | 3 (2.4) | 2 (3.8) | 1 (1.4) |
Events are shown as frequency (%).
TIA: transient ischemic attack.
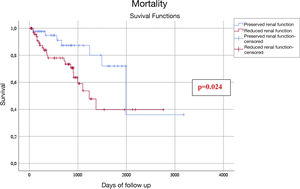
During the combined hospitalization and follow-up period there were no statistically significant differences in the annual rate of thromboembolic events in patients with preserved eGFR vs reduced eGFR (0.97/100PY vs. 4.06/100PY, P = .092), although these were numerically higher in the group of reduced eGFR; however the rate of bleeding episodes (5.67/100PA vs. 13.3/100PA, P = .033), and mortality (6.50/100PA vs. 17.2/100PA, P = .009) were higher in the group of patients with reduced eGFR. Out of the 29 deaths, 14 were due to cardiovascular causes (5 in the preserved eGFR group and 9 in the reduced eGFR group) (Table 4). The presence of a reduced eGFR was associated with an increased risk of mortality (odds ratio: 2.711; 95% confidence interval, 1.06–6.95) (Fig. 1).
In the multivariate analysis, the independent factors associated with mortality were preserved eGFR (negative association), heart failure, and diabetes mellitus (Table 5).
Independent predictors of death (all-causes) during follow-up.
| HR (95% CI) | P-value | |
|---|---|---|
| Glomerular filtration rate > 60 mL/min | 0.33 (0.13–0.83) | .018 |
| Previous heart failure | 3.25 (1.51–6.95) | .002 |
| Diabetes Mellitus | 2.64 (1.21–5.76) | .015 |
Hazard ratios (HR) and the 95% confidence intervals (CI) were calculated using Cox multivariate regression analysis. Variables included in the multivariate analysis: age, arterial hypertension, diabetes mellitus, previous heart failure, glomerular filtration rate >60 mL/min/1.73 m2, previous history of a hemorrhagic event.
The TEE follow-up was available in 95 of the 124 patients (76%). During follow-up, thrombus formation on the device was detected in 7 patients (5.64%): 2 patients with preserved eGFR (3.92%) and 5 with reduced eGFR (6.85%), with no statistical differences between the two groups groups (P = .451).
Regarding changes in renal function after the procedure, a slight but significant decrease in mean eGFR was observed in the reduced eGFR group as compared to the preserved eGFR group (3.5 mL/min/1.73 m2 [IQR −2.5 to 14.5] vs. 0.0 mL/min/1.73 m2 [IQR −4 to 4]) (P = .040).
DiscussionThe main results of this study are: 1) percutaneous closure of the left atrial appendage is associated with a high success rate and a low number of procedural complications; this is observed in both patients with reduced and preserved eGFR; 2) during follow-up, it was observed that the mortality and bleeding rates were higher in patients with reduced eGFR that also had a tendency towards a greater number of thromboembolic events, 3) during follow-up the incidence of thrombi on the device was low in both groups, but it was numerically higher in patients with reduced eGFR group, 4) the procedure was associated with a slight, but significant, decrease in the renal function in patients with reduced eGFR; there was no change in renal function in the preserved eGFR group.
Thus, LAAC is a safe procedure in the population of patients with reduced eGFR, with an overall success rate of 99.2% of cases in our study, similar to that of previous studies25–28 and higher than that of the first randomized studies,13,14 which could be explained by higher experience by the current teams performing the procedure. It also confirms the results of previous studies,25–28 regarding the rate of events during the procedure and the hospital admissions, which was very low, and without statistically significant differences in thromboembolic and bleeding events between patients with preserved and reduced eGFR. From all this we can conclude that the procedure needs to be performed by an experienced interventional cardiology team with multidisciplinary support (cardiac surgeon for possible emergencies, echocardiographist to guide the procedure, specialized nursing team, etc.).
In our study, during follow-up patients with reduced eGFR presented numerically more thromboembolic events, although it did not reach statistical significance. In addition, a higher rate of bleeding was observed in the subgroup of patients with reduced renal function (which already had a higher HASBLED at baseline). LAAC require antiplatelet therapy for at least three months after the procedure, and patients with CKD have a higher risk of bleeding,4,8 which could contribute to the higher rate of bleeding observed in these patients, especially at the beginning of follow-up. In fact, in our study, almost half of the bleeding episodes were recorded within the first three months after the procedure, similar to what was observed in the study by Genovesi et al., in which the majority of hemorrages after LAAC (6 out of 8) occurred during the first three months after the procedure.31
In our study, mortality during follow-up was higher in patients with reduced eGFR, with a rate that was 2.7 times higher than that described in previous studies,25,26 which could be explained by differences in the populations. However, it is unlikely to be related to the procedure, as seen in other studies, it is likely to be related to the worse prognosis of CKD per se.25,26
The only three published randomized multicenter studies on this treatment had a non-inferiority design, and compared the LAAC (with the Watchman™ device) vs. anticoagulation with warfarin in patients with atrial fibrillation, but without contraindication of oral anticoagulation the first two13,14; and the third study compared the LAAC with various devices vs. DOACs in patients with NVAF and a history of bleeding, thromboembolic events despite oral anticoagulation, or high risk patients (27.8%).28 As compared with our population, in the first two studies, the median age, the percentage of women, and the prevalence of hypertension and diabetes mellitus were similar, but our population had more thromboembolic and bleeding histories and a higher CHA 2 DS2 -Vasc and HASBLED scores, which is explained by the higher risk of our population. This could justify that the rate of events observed during follow-up was higher in our population, especially as compared to the PROTECT AF study.13 Our series are more comparable to those of the third randomized study, although this one excluded patients with CKD stages 4–5,28 and to the most important registries published to date on LAAC, which are the EWOLUTION and the ACP Multicentric Registry in which most of their patients had contraindications for anticoagulation and, therefore, the indication for this procedure was similar to that of our series. Baseline characteristics were similar in terms of sex, age, arterial hypertension, diabetes mellitus, and s CHA2 DS2 -Vasc score, but our patients had a higher mean HASBLED score and more thromboembolic episodes. During follow-up, we have observed a higher rate of bleeding (major and minor bleeding were collected), thromboembolic events and mortality. The most important study that compared patients with CKD with patients with preserved renal function also observed a greater increase in mortality in CKD, but not significant differences in bleeding or thromboembolic events between both groups25; similar results were observed in the study by Brockmeyer et al.30 and in the study by Xue et al., who also observed a trend towards higher mortality in the subgroup with CKD. Our results also show significant differences in the rate of bleeding between both groups and numerically more thromboembolic episodes which are worse results than previous studies in patients with CKD,25,26,30 this could be due to differences between the populations, and the fact that in our study were recorded both major and minor bleeding events (vs. only major bleeding in previous studies), also we had a higher percentage of patients with advanced CKD or on dialysis, or the timeof follow-up. Studies in patients with CKD stage 5 on hemodialysis also show that LAAC is a safe and effective procedure for this high-risk population.31–33 In fact, our study included patients who had previously been included in a multicenter study25 and in an analysis of cases in dialysis patients,32 which could partly explain the similarity of results. It would be interesting to carry out multicenter studies in our country with a close collaboration between the cardiology and nephrology departments to know the results of this technique in a larger number of patients to be able to evaluate its efficacy. In this sense, there is a Spanish observational study in patients with stage 5 chronic kidney disease on hemodialysis, which evaluates the efficacy and safety of LAAC in this population (WATCH-HD, NCT03446794).
Although patients with CKD have an increased risk of thromboembolism, renal dysfunction is not included in the CHA2 DS2-Vasc scale and, although we found no differences between the two groups, in other studies patients with decreased renal function have a higher score on this scale than those with preserved kidney function.25,26,29 Another aspect to be taken into consideration is the thrombosis on the device, since the presence of CKD is a risk factor for the formation of thrombi in the left atrial appendage. Although we did not observe significant statistical differences in the formation of thrombi on the device in both groups, patients with decreased renal function presented a numerically higher prevalence of thrombi on the device as compared to patients with preserved renal function, although this was low in both groups.
Percutaneous LAAC is an invasive procedure with more morbidity and mortality in patients with CKD.18 In addition, a decreased eGFR at baseline is the main factor leading to the deterioration of renal function after the procedure.34 In our study, patients with reduced renal function, despite not receiving more intravenous contrast, presented a worse renal function deterioration than the group with preserved renal function at discharge. This coincides with the study by Brockmeyer et al.29 who found a higher incidence of acute renal failure in the CKD group as compared to the group with preserved renal function (11% vs. 0%). It is therefore advisable to monitor renal function at baseline and after the procedure in patients undergoing LAAC, as well as to minimize or avoid the administration of iodinated contrast, especially in patients with CKD.
Among the limitations of this study is that it is an observational, retrospective study without a control group treated with OAC, and in addition, this includes relatively small sample of patients. The definition of CKD was based on the determination of the baseline eGFR and not a confirmed decrease in eGFR for at least 3 months, as recommended by the guidelines. There have been used various protocols with different duration of antithrombotic treatment after discharge, which could explain complications during follow-up, and the follow-up period of the patients has been very variable. The lack of data due to losses of follow-up and the subjective bias of collecting part of the data by telephone calls from after the first year of the procedure may have influenced the quality of the results of this study. Another limitation of the study is that the limited number of patients with CKD stages 4, 5 or 5D, in which VKA anticoagulation is associated with a high risk of bleeding and there is little evidence of its benefit, and the use of DOACs has little evidence of efficacy and safety (stage 4) or there are contraindicated (stage 5), and therefore patients with the greatest potential benefit, is limited.
In conclusion, percutaneous LAAC appears to be a safe technique, both in the population with preserved renal function and in the population with reduced eGFR, since no significant differences have been observed in intraprocedural complications or in the rate of thromboembolic events during follow-up in these two populations. However, differences were observed in the rates of bleeding and mortality during follow-up in patients with reduced renal function versus those with preserved renal function, which could be due to the CKD itself. For this reason, LAAC could be a valid option in patients with non-valvular atrial fibrillation and chronic kidney disease with a high thrombotic and hemorrhagic risk, since it would reduce thromboembolic risk, reducing the risk of bleeding associated with OAC.
Key conceptsPatients with advanced CKD have a higher prevalence of atrial fibrillation, and this is associated with a higher risk of thromboembolism, bleeding and mortality.
Evidence of the benefit-risk of VKAs and DOACs in CKD stages ≥ 4 is limited, since in general clinical trials have excluded these patients.
Percutaneous LAAC may be an alternative strategy to prevent thromboembolic risk without the risks of chronic anticoagulation in this high-risk population.
Our experience confirms that percutaneous LAAC is a valid alternative in patients with CKD, with a low risk of periprocedural complications; however, larger studies are needed, especially in patients with CKD stages 4–5, to confirm its long-term efficacy and safety.
Authorship/collaboratorsAll authors have made substantial contributions to each of the following: 1) study conception and design, or data acquisition, or data analysis and interpretation, 2) article draft or critical review of the intellectual content, 3) the final approval of the version presented.
Conflict of interestsXF is a proctor for Abbott Medical. The rest of the authors declare that they have no conflict of interest.
Please cite this article as: Benini Tapias J, Flores-Umanzor E, Cepas-Guillén PL, Regueiro A, Sanchís L, Broseta JJ, et al. Impacto pronóstico de la enfermedad renal crónica sobre el cierre percutáneo de la orejuela izquierda en la fibrilación auricular: una experiencia unicéntrica. Nefrologia. 2022;42:290–300.
This manuscript has not been published previously, except as an abstract for conferences or as a final degree project by Julia Benini.