Here we describe the case of a 27-year-old female patient with a history of hypertension and cutaneous lupus, who was admitted for a kidney biopsy because she had proteinuria (2081mg/g), microhaematuria (10–20 RBC/field) and mild functional impairment (Cr 1.3mg/dl). The procedure was complicated with a severe perirenal haematoma, which required selective embolization and, subsequently, fever, which was attributed to the procedure and reabsorption of the haematoma; the microbiological tests were negative. The patient developed anaemia (Hb 8g/dl) with normal WBC and platelets. Autoimmunity lab results were: ANA test was positive (1/160); the anti-DNA, positive (33U/ml); and the ENA, SSA, SSB, U1RNP, Sm and SCL70, negative. The lupus anticoagulant (2.3) and the IgG anticardiolipin antibodies (253U/ml) were positive, with decreased C3 levels (70mg/dl). The kidney biopsy showed a type III lupus nephritis. There were only 3 glomeruli (2 with an increased mesangial matrix and one with focal and segmental sclerosis/hyalinosis) with no “wire loop” lesions or glomerular crescent formations. Immunofluorescence showed deposits in the mesangium and the glomerular basement membrane of high intensity for IgG, C3 and C1Q, and mild/moderate intensity for IgA, IgM and C4.
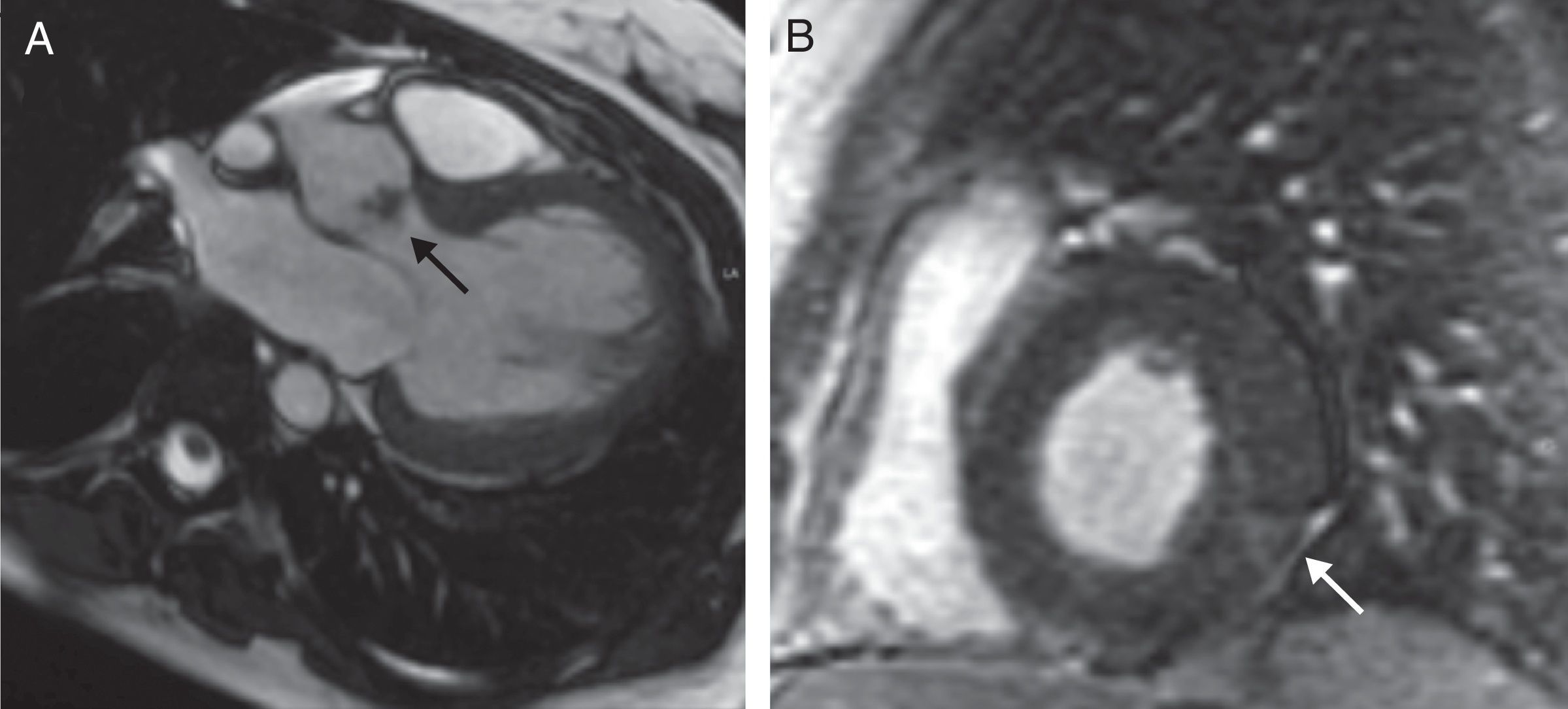
The patient had cardiac murmur in the auscultation and the fever persisted, so an echocardiogram was performed that showed a bicuspid aortic valve with a vegetation s on the free edge of its 2 leaflets causing a severe insufficiency. Although it was unlikely that this lesion was caused by an infection, owing to the negative result on the blood cultures, a cardiac MRI with contrast dye was performed to clarify the diagnosis. In this examination, in addition to the vegetation there was subepicardial enhancement inferolateral, suggestive of a vasculitic process (Fig. 1), with no evidence of abscess, pericardial effusion or other complications.
Cardiac MRI with contrast dye. (A) Fast imaging employing steady-state acquisition (FIESTA) sequence in left ventricular (LV) outflow tract, showing a vegetation on the aortic valve (arrow). (B) Short-axis delayed gadolinium-enhanced sequence of the LV, showing an image of subepicardial enhancement at the level of the inferolateral segment suggestive of a vasculitic process (arrow).
With a diagnosis of Libman-Sacks endocarditis (LSE), anticoagulant therapy was started, combined with steroids (1mg/kg/day) and mycophenolate (MMF) up to a dose of 1g/12h. After one, she has normal kidney function, the aortic insufficiency has improved to a more moderate gradient.
Although the use of steroids in the treatment of systemic lupus erythematosus (SLE) has caused a significant reduction in the incidence of LSE, its current frequency could be up to 10–35% of cases.1,2 However, its clinical manifestations tend to be mild, and so it often happens unnoticed.1 LSE is usually associated with a long duration of the disease and with the existence of anticardiolipin antibodies (aCL) and antiphospholipid syndrome (APS).1,3 The “two-hit hypothesis”, described by Bordin et al.,4 suggests that the pro-thrombotic state generated by aCL (first event) would promote (after a second event such as heart surgery, pregnancy, etc.) the formation of a blood clot on a previously inflamed valvular endothelium.3–5 In the present case, at least from a renal point of view, the disease was not of long duration, but, based on the two-hit hypothesis, one might speculate that, the presence of an aCL-positive during lupus flare-up, plus the damage caused by the haematoma and subsequent embolization, could trigger endocarditis on a valve that is, susceptible because a bicuspid morphology.
This case also illustrates the difficulty of a differential diagnosis vs other valvular malformations or infective endocarditis itself, which may be colonizing on a previous LSE lesion. Although LSE is usually located on the mitral valve, it can also occur on the aortic (as in our case), the tricuspid (less than 10% of cases) and the pulmonary valve.1,3 Because there was no characteristic location or echocardiographic image, the MRI was very useful in clearing up the diagnosis, a finding also documented by other authors.6
Regarding treatment, surgery is reserved for cases of severe valvular dysfunction or with large mobile masses with high risk of embolization.7 Although steroid treatment may reduce lesions, it is generally not sufficient for eradicating the embolic risk, so patients should be anticoagulated.8 In our case, and also given the presence of APS and lupus nephritis, treatment with MMF was proposed, with a good renal response and a striking heart response as well, and with a significant improvement of the lesions.
In conclusion, LSE should be included in the differential diagnosis of a patient with SLE and fever, especially if the test is positive for anticardiolipin antibodies and there is a previous triggering factor. In the absence of specific markers, MRI is an important tool for elucidate the diagnosis. Although valvular dysfunction is rare, when it occurs, treatment with steroids and MMF may reduce the lesions and postpone the need for valve replacement. However, given the embolic risk involved, maintaining anticoagulation with close clinical and imaging observation is essential.
Please cite this article as: Gómez-Larrambe N, González-Tabarés L, Millán B, Cobelo C, Armesto V, Pousa M, et al. Endocarditis de Libman-Sacks: una manifestación frecuentemente inadvertida. Nefrologia. 2017;37:217–219.