The increase in the number of patients on the kidney transplant waiting list has led to an attempt to increase the number of potential donors by incorporating candidates that previously would not have been considered optimal, including donors after cardiac death (DCD) and those with “expanded” criteria (ECD). Recipients of controlled DCD (cDCD) grafts suffer more delayed graft function (DGF), but have a long-term evolution comparable to those of brain-dead donors, which has allowed an increase in the number of cDCD transplants in different countries in recent years. In parallel, the use of cDCD with expanded criteria (cDCD/ECD) has increased in recent years in different countries, allowing the waiting list for kidney transplantation to be shortened. The use of these grafts, although associated with a higher frequency of DGF, offers similar or only slightly lower long-term graft survival than those of brain death donors with expanded criteria. Different studies have observed that cDCD/ECD graft recipients have worse kidney function than cDCD/standard and DBD/ECD. Mortality associated with cDCD/ECD graft transplantation mostly relates to the recipient age. Patients who receive a cDCD/≥60 graft have better survival than those who continue on the waiting list, although this fact has not been demonstrated in recipients of cDCD/>65 years. The use of this type of organ should be accompanied by the optimization of surgical times and the shortest possible cold ischemia.
El incremento en el número de pacientes en lista de espera de trasplante renal ha llevado a intentar aumentar el número de posibles donantes incorporando candidatos que anteriormente no se habrían considerado óptimos, incluyendo entre estos a los donantes de pacientes de asistolia (DA) y aquellos con criterios “expandidos” (DCE). Los receptores de injertos de DA controlada (cDCD) sufren más función retrasada del injerto, pero presentan una evolución a largo plazo equiparable a los de donantes de muerte encefálica, lo que ha permitido un aumento en el número de trasplantes de cDCD en distintos países en los últimos años. De forma paralela, el uso de cDCD con criterios expandidos (cDCD/DCE) se ha incrementado en los últimos años en diferentes países, permitiendo acortar la lista de espera del trasplante renal. El uso de estos injertos, aunque se relaciona con una mayor frecuencia de función retrasada del injerto, ofrece supervivencias del injerto a largo plazo similares o solo ligeramente inferiores a las de los donantes de muerte encefálica con criterios expandidos. Distintos estudios han observado que los receptores de injertos cDCD/DCE tienen peor función renal que los cDCD/estándar y que los donantes de muerte encefálica/DCE. La mortalidad asociada al trasplante de injertos de cDCD/DCE se relaciona principalmente con la elevada edad del receptor. Los pacientes que reciben un trasplante renal de cDCD/≥60 años presentan mejor supervivencia que los que continúan en la lista de espera, aunque este hecho no se ha demostrado en los receptores de cDCD/>65 años. La utilización de este tipo de órganos debe llevar pareja la optimización de los tiempos quirúrgicos y el menor tiempo de isquemia fría posible.
The increase in the number of patients on the kidney transplant waiting list despite the high donation rates in different countries, has led to make efforts to increase the number of potential donors by incorporating candidates who previously would not have been considered optimal. These potential donors include donors from patients with asystole (DCD), expanded criteria donors (ECD), donors with acute renal failure, diabetic donors or donors with viral infection, etc.1 DCEs were defined in 2002 by Port et al. based on data from the American transplant registry (SRTR), as those donors from deceased patients who had a greater than 1.7-fold risk of renal graft loss. These donors were all patients aged ≥ 60 years and those ≥ 50 years with at least two of the following characteristics: final creatinine > 1.5mg/dL, high blood pressure and/or cerebrovascular accident (CVA) as cause of death.2 Subsequent studies have validated this definition, since in general, death brain donors (DBD) with expanded criteria increased the subsequent risk of graft loss in the expected range of 1.7 times.3 Despite the number years that have elapsed since its definition, the validity of these criteria has also been confirmed by a prospective study in a current population of kidney transplant recipients.4 The incorporation of the KDRI criteria in US donors has made it possible to “measure” the “quality” of the potential donor in a more gradual manner through a continuous variable that overcomes some of the disadvantages of using a dichotomous definition, such as DCE, on the quality of the donor.5 Although its relationship with the risk of graft failure has been confirmed (HR 1.03 [95% CI; 1.01–1.05]; p=0.014) in the Spanish population, the use of the KDRI criteria has not been generalized in our setting and their extrapolation does not seem advisable given the differences in organ harvesting strategies between the US and European countries.6,7
Using kidney grafts from ECD donors or from donors with high KDRI values has been shown to be beneficial for kidney transplant recipients, being especially beneficial if the recipient is properly selected. In this regard Merion et al. showed that receiving a graft from a ECD donor reduced the recipient's mortality by 17% compared to remaining on the transplant waiting list or subsequently receiving a transplant from a conventional donor. The risk of death already decreased after the eighth month with respect to remaining on the waiting list and was especially beneficial in recipients over 40 years of age, diabetics and in organizations with long average waiting times.8 Similarly, Massie et al. demonstrated in 184,277 SRTR patients that receiving a graft from a donor with high KDRI values, even above 90, also improved patient survival.9 The benefit of receiving a kidney transplant has been shown to be favorable, even receiving a graft from a donor over 75 years of age.10
In parallel, in recent years there has been a significant increase in the number of donors after circulatory death (DCD), also called non-heart beating donors, mainly controlled Maastricht III, in different countries. In the United Kingdom, the percentage of donors after circulatory death (DCD), mostly controlled cDCD, increased six times between 2004 and 2013; In the Netherlands, 43% of the kidney transplants performed between 2000 and 2017 came from cDCD 11,12. Similarly, in Spain, cDCD accounted for 24% of all transplants performed in 2018 and 26% of transplants from deceased donors. This increase is due to evidence that cDCD grafts, despite the inherent risk of associated warm ischemia, have the same long-term graft survival as death brain donnors (DBD) grafts. Thus, in the United Kingdom study and in the Dutch registry study, recipients of cDCD grafts suffered a significantly higher rate of delayed graft function (DGF) (49% vs. 24%, p<0.0001 in the British study; 42 vs. 17%, p<0.0001 in the Dutch) and a slight increase in the frequency of “primary non-function” (PNF) (4 vs. 3%, p=0.02 in the British study; 10 vs. 8%, p=0.0001 in the Dutch), but had the same graft survival (in the British study, at three years the HR: 1.18, 95% CI; 0.95–1.47, p=0.13) and patient survival (at three years HR: 0.98, 95% CI; 0.75–1.30, p=0.93) than conventional donors.12,13,14 A recent meta-analysis of 12 studies including 6008 DCD and 13,129 DBD transplants found no significant differences in graft survival at one, three, five, and 10 years.15 The use of DCD has also been shown to reduce mortality significantly, by 56%, as compared to those who continued on the waiting list, even if they subsequently received a conventional DBD graft.16
Both ECD and DCD grafts are discarded for transplantation at a higher rate than grafts from conventional donors. Thus, in the study by Marrero et al., the risk of discarding DCD kidneys was 2.5 times higher than conventional donors, and the risk of discarding grafts from donors over 50 years of age was more than three times.17 If both situations occur together, up to 51% of DCD/SCD kidneys are discarded.18 Despite this, both the percentage of DCD and ECD and the age of the donors have been increasing in recent years. In the United Kingdom, between 2001 and 2012, 20% of donors were cDCD and, of these, more than 40% met ECD criteria and they were older than 60 years in 2013.11 In Spain, the progress has been similar, with more than 50% of non-heart beating donors currently over 60 years of age.19 Despite the fact that these results have made it possible to shorten the waiting list,20 the combination of both factors (DCD and ECD) in the same donor could modify the results of the transplant. The objective of this review is to summarize the previous experience published with the use of cDCD with ECD and/or of high age and the variables that can contribute to modifying the course of these transplants.
Donation in controlled donors after circulatory death with expanded criteria donorsThe most relevant studies analyzing the results of using cDCD/ECD grafts were performed using SRTR data (Table 1). Singh et al. analyzed 67,816 deceased-donor transplants, including 562 with expanded criteria asystole (AD/ECD) performed between 2000 and 2009. As expected, the authors found a higher frequency of PNF (2.9 vs. 0.9%) and DGF (53.3 vs. 39.6%) when using ECD grafts compared to standard grafts in the group of DCD recipients. This increased risk was also observed in recipients of DBD, both in PNF (1.5 vs. 0.7%) and in DGF (30.5 vs. 21.3%), such that the increased risk of PNF and DGF using ECD grafts was only slightly higher in DCD recipients, without finding that the cDCD/ECD interaction was significantly worse than DBD/ECD.21 Regarding long-term outcomes, the findings were similar. Receiving a DCD/ECD graft increased the risk of death censored graft survival as compared with DBD/ECD grafts, but this increased risk was not disproportionately greater than that experienced by those who received a DCD/SCD graft in relation to those who received a DBD/SCD graft. The results of this analysis were similar if, instead of using the expanded criteria, the donors were classified according to KDRI quintiles.21
Studies that compare the results of transplants from controlled donors after circulatory death and expanded criteria, older than 60 years or older than 65 years.
| Authors | Country | Study design | n | Results | Conclusions | |
|---|---|---|---|---|---|---|
| Singh, 2013 21 | USA | Registry of transplant recipients «SRTR» | 67,816 (5402 DCD) | ECD/DCD vs. ECD/DBD | + PNF | ECD/DCD slightly lower than SCD/DCD but acceptable results. |
| + DGF | *The slight increased risk of graft loss associated with DCD is not significantly increased with ECD. | |||||
| - graft survival* | ||||||
| ∼ patient survival | ||||||
| Nagaraja, 2015 22 | UK | [Retrospective single center | 359 (112 cDCD) | ECD/cDCD vs. SCD/cDCD | =PNF | ECD/cDCD survival similar to SCD/cDCD and ECD/DBD and eGFR less than 2 years. |
| =DGF | ||||||
| =graft survival | ||||||
| =patient survival | ||||||
| - eGFR 2 years | ||||||
| ECD/cDCD vs. ECD/DBD | + DGF | |||||
| =graft survival | ||||||
| - eGFR 2 years | ||||||
| Locke, 2007 25 | USA | Multicenter registry | 78,174 (2562 DCD) | ≥ 60/cDCD vs.≥60/DBD | + DGF | cDCD recipients <50 years have long-term graft survival like SCD/DBD |
| - graft survival | cDCD recipients >50 years of age have 5-year graft survival comparable to ECD/DBD. | |||||
| Favi, 2018 26 | UK | Single center observational | 152 (12 cDCD) | ≥ 60/cDCD vs.<60/cDCD | =PNF | Graft recipients ≥ 60/cDCD have higher |
| ≥ 60/cDCD vs.≥60/DBD | + DGF | DGFs and worse patient and graft survival than those with < 60/cDCD. | ||||
| - graft survival | Graft recipients ≥ 60/cDCD have more DGF, but the same patient and graft survival as those ≥ 60/DBD. | |||||
| - patient survival | ||||||
| - eGFR at 5 years. | ||||||
| =PNF | ||||||
| + DGF | Graft recipients of ≥60/cDCD have worse renal function. | |||||
| =graft survival | ||||||
| =patient survival | ||||||
| - eGFR at 5 years | ||||||
| Pérez-Sáez, 2019 28 | Spain | Spanish multicenter retrospective «GEODAS» | 561 cDCD | >65/cDCD vs.≤65/cDCD | =DGF | The results for >65/cDCD are similar to those obtained with ≤ 65/cDCD. |
| =PNF | The poorer patient survival of donor treatment recipients ≥65 years of age with cDCD is due to the older age of the recipient. | |||||
| =graft survival | ||||||
| - patient survival (but related to receptor age) | ||||||
| - eGFR 1st year | ||||||
| Peters-Sengers, 2017 30 | Netherlands | Dutch Transplantation Registry | 3597 (1434 cDCD) | ≥ 65/cDCD vs.<65/cDCD | =PNF | Recipients of donors ≥ 65 years cDCD have similar graft and recipient survival outcomes to donors ≥ 65 years DBD. |
| =DGF | Elderly recipients of donors ≥65 years of cDCD have similar graft survival, but higher mortality than elderly recipients of donors <65/cDCD | |||||
| =graft survival at 5 years | ||||||
| - patient survival | ||||||
| - eGFR | ||||||
| ≥ 65/cDCD vs.≥65/DBD | + PNF | |||||
| + DGF | Recipients >65 years of donors ≥65/cDCD have lower eGFR. | |||||
| =graft survival at 5 years | The survival of the elderly recipient of a transplant from a donor ≥ 65/cDCD is similar to that of elderly patients who continued on the dialysis waiting list. | |||||
| ∼ patient survival | ||||||
| - eGFR | ||||||
| Buxeda, 2020 29 | Spain | Retrospective Unicenter | 213(87 cDCD) | ≥ 65/cDCD vs.<65/cDCD | =NFP | Treatment outcomes of donors ≥65 years of cDCD have similar results to those of donors ≥65 years of DBD and donors of <65 years/cDCD. |
| =DGF | The worse 3-year patient survival of tx recipients from donors ≥65 years of age with cDCD is due to the older age of the recipient. | |||||
| =graft survival | ||||||
| - patient survival at 3 years | ||||||
| ≈ eGFR at 3 years | ||||||
| ≥ 65/cDCD vs.≥65/DBD | =PNF | |||||
| =DGF | ||||||
| =graft survival | ||||||
| =patient survival | ||||||
| ≈ eGFR at 3 years | ||||||
ECD: expanded criteria donors; SCD: standard criteria donor; cDCD: controlled donors after circulatory death; DBD: death brain donors; DGF: delayed graft function. SCD: standard criteria donor.
Subsequent studies in different populations have detected similar findings. Thus, Nagaraja et al. have reported that, although they suffer more frequently from DGF (72 vs. 35%, p<0.001), the evolution of cDCD/ECD grafts was similar to that of DBD/ECD grafts, with a comparable 2-year graft survival (81% vs. 79%, p=0.77) (Table 1).22 Interestingly, whenever there were ECD grafts used, added to the effect of asystole, renal function is worse. Nagaraja et al. found that the estimated glomerular filtration rate at the second year was significantly worse in the group of recipients of DCD/ECD transplants (33mL/min) than in the group with DCD/SCD (54mL/min) and with DBD/ECD (47mL/min, p=0.007).22
Despite acceptable data obtained on both graft and patient survival with DCD/ECD kidneys, it is not known with precision whether receiving an organ with these characteristics offers advantages in terms of patient survival compared to remaining in the waiting list on regular dialysis. The only study that has analyzed this aspect, has observed a decreased risk when receiving a kidney graft with these characteristics but, without reaching statistical significance (HR 0.61, 95% CI; 0.31–1.19, p=0.15), so at this point it cannot be established that receiving an DCD/ECD kidney improves patient survival.16
Donation after circulatory death with donors older than 60 yearsAlthoug the remaining criteria that define ECD (hypertension, final creatinine, and death from stroke) are also related with DCD graft survival in addition, it has been demonstrated that the greater the number of criteria, the worse the outcome of the transplant.23,24 However, the factor that most influences the evolution of DCD grafts is the age of the donor. In fact, Locke et al. observed, using SRTR data, that donor age was the only variable that affected graft survival, increasing the risk of graft loss by 78% with DCD≥60 years and 48% with DCD between 50 and 59 years (table 1)25. Studies from the British registry have also shown that as the donor ages, graft survival decreases. The adjusted risk of graft loss in DCD between 40–59 years was 1.73 (95% CI; 1.20–2.49) and, above 60 years it was 2.76 (95% CI; 1.87–4.08); this effect of the donor's age is only slightly higher, without statistical significance, than that shown in the DBD (40–59 years HR: 1.60, 95% CI; 1.22–2.01; ≥ 60 years HR: 2.16, 95% CI, 1.63–2.86).14
Longer-term studies have reported similar findings. In a single-center British study, cDCD grafts over 60 years of age showed more PNF (12.5% vs. 1.4%, p=0.021), plus DGF (70 vs. 47.2%, p=0.029) and worse five years survival of both graft (63 vs. 83%; p=0.001) and patient (66 vs. 85%; p=0.014) as compared to cDCD donors under 60 years of age (Table 1). And, in the group of donors ≥ 60 years receiving a cDCD graft was associated with a higher rate of DGF (70 vs. 37.5%, p=0.007) and an comparable survival of both, the graft (63 vs. 69%, p=0.518) and the patient (66 vs. 67%, p=0.394). As previously mentioned, this study also confirmed that cDCD/≥60 donors had worse kidney function than death brain donors (34mL/min vs. 41mL/min, p=0.029).26
Despite that DCD recipients aged ≥ 60 years experienced highest rate of DGF, worse kidney function, and graft survival than younger DCD recipients, it is well documented that patients on the waiting list who receive a cDCD graft aged ≥ 60 years show improved survival than the remaining on the waiting list, even if they receive a transplant later on. The analysis of the North American SRTR registry recently performed has noticed that recipients of a cDCD renal graft ≥ 60 years have shown a 48% lower risk of death (HR 0.52, 95% CI; 0.46−0.55), p<0.001) than those who remained in the waiting list (three-year mortality 12.2 vs. 22.0%; six-year mortality 29.8 vs. 36.8%, p=0.003).27
Donation in controlled donors after circulatory death older than 65 yearsThere are few data about the influence of cDCD older than 65 years on the kidney transplant outcomes. Of 561 cDCD transplants included in the GEODAS study cohort, 135 had received a donor kidney graft > 65 years (Table 1). In this study, grafts from cDCD > 65 years did not show higher incedence of more PNF (3.7 vs. 3.1%, p=0.71) or DGF (55.4 vs. 46.7%, p=0.09), but they showed worse kidney function (49mL/min vs. 58mL/min, p<0.001) and higher first-year mortality (6.9 vs. 1.9%, p=0.004). Despite of worse renal function, these grafts of cDCD over 65 years showed the same death-censored graft survival. After a multivariate analysis, it was shown that the worse survival of the patient who received a cDCD >65 years graft was exclusively related to recipients. This finding is consistent with the observations previously described by other authors.26,28 Similar findings have been confirmed in the retrospective study performed in the Hospital del Mar in Barcelona in 87 cDCD recipients (46≥65 years vs. 41<65 years) (Table 1).29
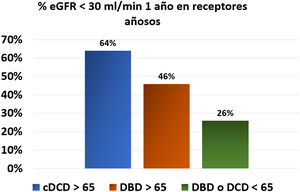
In the same line, the Dutch registry studies provided relevant information on the outcomes of this type of donor (Table 1). This study found no significant differences (HR 1.57, 95% CI; 0.79–3.11, p=0.20) in graft survival between cDCD > 65 years and those with DBD<65 years, while the risk of recipient death was significantly higher, with a 5-year patient survival of 51% in DCD≥65 years, 55% in DBD≥65 years, and 69% in donors < 65 years both DCD and DBD. In addition, renal function at one year was worse in the group receiving cDCD ≥ 65 years. Thus, 64% of cDCD grafts ≥ 65 years had an estimated glomerular filtration rate lower than 30mL/min, as compared with only 46% of grafts from DBD ≥ 65 years and 26% if the donors were < 65 years (Fig. 1).30 No significant differences were found in 5 the five year mortality by comparing the evolution of potential elderly recipients over 65 years who entered on dialysis (60%) versus those that received a cDCD ≥ 65 years graft (65 %) or DBD≥65 years (61%). Therefor we are not allowed to conclude that recipients > 65 years improve their survival by receiving a transplant from a donor older than ≥ 65 years (both cDCD or DBD).30 Analysis of data from Addenbrooke’s Hospital in Cambridge also suggests that the mortality of transplant patients over 65 years of age, who received predominantly cDCD grafts (63%) with a mean age of 67 years, was similar (11%) to being maintained on the waiting list (8%).20
Percentage of patients with an estimated glomerular filtration rate <30mL/min one year after transplant according to the characteristics of the donor from the Dutch registry.30
% eGFR <30mL/min one year after transplant in aged recipients. DCD: Donors cardiovascular death. DBD: Donors brain death.
There are no conclusive data on the role of hemodynamic changes during agonal phase on the results of cDCD. Peters-Sengers et al. showed that the longer the duration of the agonal phase the greater the risk of DGF. An increase of 7–20min in the period of systolic blood pressure (BP) < 80mmHg was associated with a 2.19 times increase the risk of DGF.31 Other donor parameters, such as the duration of warm ischemia time, hypotension before extraction or the slope of oxygen saturation, have been related to the initial and/or long-term evolution of the graft.32,33 A prolonged functional warm ischemia time (FWIT) is strongly associated to poorer graft survival. In current practice, FWIT>60min is enforced as a contraindication to renal harvesting in most centers, but the strict upper limit of FWIT remains controversial. Several experiences in centers with a high volume of DCD transplants with excellent results suggest that the upper limit time could be increased to 2−4h for renal DCD.34–36
Perhaps, the cold ischemia time (CIT) is the modifiable factor that may have the greatest influence on the results in cDCD.12,14 Data from the GEODAS study confirmed that cold ischemia greater than 14h increased the risk of PNF (OR 4.4, 95% CI; 1.3–14.4) and DGF (1.6, 95% CI; 1, 1–2,3) independently of other variables.37 In the long term, a prolonged CIT influences renal graft loss, but mainly in cDCD recipients and not in those of DBD. Using data from the British registry, Summers et al. highlighted that the risk of graft loss was higher in those who had a CIT between 12 and 18h (HR 1.53, 95% CI; 1.03–2.30) compared with those who had less than 12h and it was even higher higher in those with cold ischemia time greater than 24h (HR 2.36, 95% CI; 1.39–4.02), but this effect was detected as relevant only in cDCD.14 Although an interaction between the effect of cold ischemia and donor age or expanded criteria was not detected, it seems reasonable to try to shorten ischemia times to optimize the results of cDCD/SCD grafts. In fact, and despite the increase in the age and KDRI of donors over the years, the improvement observed in the results of DCD transplantation during the last decade in the Dutch registry has been related to the progressive reduction in the average CIT from 20 to 15h.38
According to the means of preservation in situ before extraction, the data from the GEODAS study shows that in Spain rapid laparotomy with cold static preservation was performed in 61%, antemortem cannulation and cold perfusion in 16% and, in the rest, normothermic perfusion with extracorporeal oxygenation.37 The possible advantages of normothermic perfusion with extracorporeal oxygenation would be to limit ischemic damage by restoring the energy substrates of the cells and, in addition, to allow that the extraction procedure be performed without urgently reducing surgical kidney damage frequently observed in rapid surgery.39 In the context of uncontrolled DCD, normothermic perfusion with extracorporeal oxygenation has been shown to improve renal graft survival by decreasing the risk of PNF by more than fourfold40,41. Although there are no comparative studies in cDCD, published studies suggest that if normothermic regional perfusion is used the rate of DGF is lower (18–40%) than in cases of super-rapid extraction (48.5% in the British registry and > 60% in Dutch)11,30,39. The experience of the Hospital Marqués de Valdecilla in 27 DCD with NRP has shown positive results with a low rate of DGF (27%), and progress of renal function indistinguishable from that of a group of brain-dead donor transplants and without differences in graft survival at one year between cDCD with preservation with normothermic regional perfusion 92 vs. 97% with brain death (p=0.315).42 The use of normothermic regional perfusion with oxygenation devices could minimize ischemic damage before kidney recovery and increase the availability of kidneys obtained from cDCD with prolonged FWIT.
In Spain, during the period 2012–2017, the perfusion machine was used in approximately 12% of the cDCD kidneys as an ex situ preservation method, and the remaining grafts were cold preserved. The use of one or another preservation method did not modify the percentage of organs finally used for transplantation.19 There is no evidence on whether or not the perfusion machine should be used in DCD grafts; this justifies the differences in its use, 25% in the United Kingdom and 70% in the USA.43 Many studies performed with both, grafts from DBD and from DCD, have compared the post-transplant outcome of organs preserved in cold or with a perfusion machine, and often results have been discordant, even in randomized studies.44–46 Jointly almost all studies have observed that the use of NRP reduces the rate of DGF (OR 0.56, 95% CI; 0.36−0.86, p=0.008), but it does not decrease the frequency of PNF, or improves graft or patient survival or renal function.47 A recently published meta-analysis confirms these conclusions in the group of DCD transplant studies. Thus, in these transplants, the use of a perfusion machine as a preservation method reduced the frequency of DGF by 25% (RR 0.75, 95% CI; 0.64−0.87, p=0.0002), being necessary to put 7.3 grafts in a perfusion machine to avoid an episode of DGF. However, use of the perfusion machine was not found to reduce primary non-function or improve long-term patient or graft survival.48 Although many of the included studies were randomized, one criticism has been that the data analysis was not done on an intention-to-treat basis. For that reason, potentially worse grafts that were randomized to the NRP group but were cold storaged in the end, were included within this group for final analysis, so the results of cold perfusion were worse. A recent multicenter prospective randomized British study, analyzed on an intention-to-treat basis, has shown no advantages in DGF with machine perfusion in cDCD grafts (IRF cold 62.8 vs. 58.8% in machine, p=0.69).43
Some studies support the idea that the perfusion machine limits the damage induced by prolonged cold ischemia,49 which could be particularly interesting for DCD/ECD kidney grafts. Cantafio et al. reported that, compared to cold preservation, perfusion machine reduced the rate of DGF when DCD donors were older than 60 years (OR 0.76, p=0.02) and improved graft survival at three months in older than 50 years (OR 0.61, p=0.02).50 Similarly, using data from the US SRTR transplant registry, Sandal et al. observed that, although the use of the perfusion machine did not reduce DGF in DCD/ECD, it increased long-term survival in this group of transplant recipients.51
The use of a perfusion machine has also been implemented as a criterion to decide whether or not to use the organ in a large number of centers. A low flow (<80mL/min) or a high resistance index (> 0.4mmHg/mL/min) are criteria for not accepting a potential kidney graft. These two parameters are related to the characteristics of the donor, so that, in ECD donors, the flow is lower and the resistance index are higher.52 Although it is a common practice, it has not been shown that an absolute cut-off point of these parameters may be established to decide whether to accept or discard an organ.53 In the study by Jochmans et al. the final resistance index was an independent risk factor, as a continuous variable, of PNF, DGF and worse survival at one year, but with a low predictive value on the evolution of the graft with an AUC-ROC for DGF of only 0.58.54
Other characteristics that may influence the outcome of cDCD/ECD transplants are whether the recipient is a retransplant, the number of incompatibilities, or the preimplantation histology of the organ. The use of cDCD grafts in patients who had previously undergone transplantation is associated with a higher rate of PNF (3% vs. 7%) and worse renal graft survival (HR 2.74, 95% CI; 1, 96–3.82), although it has not been analyzed what would influence if, in addition, the donor also had expanded criteria.23 On the other hand, preimplantation histology is frequently used in many centers to select or discard organs for transplantation or even to place double transplants,55 however, the correlation of histological data with post-transplantation evolution is poor.56 In the United Kingdom, a prospective randomized study called “PITHIA” is being carried out in potential donors over 60 years of age, including cDCD, to analyze whether the pre-implantation histological study helps to increases the number and improves the post-transplantation evolution of grafts from elderly donors.57
Finally, in relation to the immunosuppression to be used in DCD/ECD transplants, the British guidelines recommend the use of induction in all DCD graft recipients. This recommendation is based on the fact the there is higher risk of rejection associated with DGF.58 As far as what would be the best induction therapy in cDCD/SCD transplants, the results of the GEODAS study showed that induction with basiliximab increases the risk of DGF by 47% as compared to induction with thymoglobulin37. Similar data have been provided in the aforementioned British study, in which induction with thymoglobulin was associated with less graft loss compared to induction with basiliximab (HR 0.503, 95% CI; 0.269−0.940, p=0.031), independently of other variables such as the age of the donor26.
In many centers the onset of calcineurin inhibitors is delayed or the dose is reduced to avoid or shorten the duration of DGF; however the benefits of this strategy have not been confirmed in different studies, therefore the British guidelines just mention this therapeutic option.58 In a study conducted in our center, we found no differences in the rate of DGF or in the evolution of renal function among patients with a delayed versus the initial introduction of tacrolimus (27% vs. 23%, p=0.795)59. Interestingly, avoiding calcineurin inhibitors using belatacept has been shown to be advantageous in this group of patients. In the BENEFIT-EXT study, the use of belatacept in DCD transplants was shown to be especially beneficial compared to cyclosporine, with greater graft survival and lower frequency of DGF (83% with cyclosporine, 47% in the low-dose belatacept group) and with better renal function with both DCD and ECD.60 The generalization of the use of belatacept in these patients has been limited by the lack of comparative studies with tacrolimus and by the limitations of its prescription, so a definitive recommendation cannot be made for its use in cDCD/ECD transplants.
To conclude, the use of DCD/ECD donors has increased in recent years in different countries, making it possible to shorten the kidney transplant waiting list. The use of these grafts, although associated with a higher frequency of DGF, offers similar or only slightly lower long-term graft survival than that of DBD donors with expanded criteria. Different studies have observed that cDCD/ECD graft recipients have worse renal function than cDCD/SCD grafts and DBD/ECD donors. Mortality associated with cDCD/ECD graft transplantation is mainly related to the high age of the recipient. Patients who receive a renal transplant from cDCD/≥60 years have better survival than those who remain on the waiting list, although this fact has not been demonstrated in recipients of cDCD/>65 years. The use of this type of organ must lead to the optimization of surgical and cold ischemia times.
FinancingThis work has been sponsored by the Ministry of Science and Innovation, the Carlos III Health Institute and the European Regional Development Fund (Research Network for Kidney Diseases “RedInRen” RD16/0009/0027) and Networks for Cooperative Research Health Results (“RICORS2040” RD21/0005/0010).
Conflict of interestsThis review was partially exposed at a meeting of the Prometheus group in 2019.
Please cite this article as: Barreda Monteoliva P, Redondo-Pachón D, Miñambres García E, Rodrigo Calabia E. Resultados del trasplante renal con donante en asistolia controlada expandido. Nefrologia. 2022;42:135–144.