High dose methotrexate (HD-MTX) based chemoimmunotherapy is a central part of the standard approach to treatment of primary central nervous system lymphoma (PCNSL). Renal dysfunction leads to delayed MTX complete elimination and critical MTX concentration. Despite the recommendations, hemodialysis status should not exclude HD-MTX.
We report the case of a 64 years old woman on chronic hemodialysis with PCNSL successfully treated with HD-MTX-based chemoimmunotherapy with an adjusted dose of 100mg/m2, instead of the usual dose of 3500mg/m2, and daily hemodialysis started 24h later. The patient had no significant toxicity and was in complete remission at 1 year after the end of the treatment.
We argue that ESRD is not an absolute pitfall to the use of HD-MTX for hematological malignancies. Experts should consider the use of adjusted dose at 100mg/m2 as a viable therapeutic modality in ESRD patients.
La quimioinmunoterapia basada en una dosis elevada de metotrexato (HD-MTX) es una parte central del enfoque terapéutico estándar del linfoma primario del sistema nervioso central (PCNSL). La insuficiencia renal causa la demora de la eliminación completa de MTX, así como la concentración crítica del mismo. A pesar de las recomendaciones, el estatus de hemodiálisis no debería excluir la HD-MTX.
Reportamos el caso de una mujer de 64 años con PCNSL y tratamiento de hemodiálisis crónica que fue exitosamente tratada con quimioinmunoterapia basada en HD-MTX con una dosis ajustada de 100 mg/m2, en lugar de la dosis habitual de 3.500 mg/m2, iniciándose la hemodiálisis diaria al cabo de 24 h. La paciente no reflejó toxicidad significativa y experimentó remisión completa al cabo de un año desde la finalización del tratamiento.
Nosotros argumentamos que la enfermedad renal en etapa terminal (ESRD) no constituye un escollo en absoluto para utilizar la HD-MTX para neoplasias hematológicas. Los expertos deberían considerar el uso de una dosis ajustada a 100 mg/m2 como modalidad terapéutica viable en los pacientes de ESRD.
High-dose methotrexate (HD-MTX) is the cornerstone of the chemotherapy treatment of the primary central nervous system non-Hodgkin lymphoma (PCNSL).1 Methotrexate is an antimetabolite interfering with the metabolism of folic acid, thereby blocking purine synthesis. HD-MTX defined by doses ≥500mg/m2, most often from 1 to 4g/m2, are required to cross the blood–brain barrier and achieved therapeutic level in the central nervous system.
MTX is mainly excreted by the kidneys, and delayed drug clearance may lead to toxicity.2 Toxicity is dependent on both duration of complete elimination from the body and serum MTX concentration (MTXc). Therapy drug monitoring is mandatory to guide folinic acid rescue and prevent severe toxicities.
Because renal dysfunction conduces to delayed MTX complete elimination and critical MTXc, end-stage renal disease (ESRD) is considered as a contra-indication for HD-MTX.
Nevertheless the drug is effectively cleared through hemodialysis,3 and drug monitoring is easily available. However, data is missing in these particular patients.
We report a case of a hemodialysis patient with PCNSL successfully treated by 100mg/m2 adjusted HD-MTX-based chemoimmunotherapy regimen.
A 64-year-old woman ongoing chronic hemodialysis for 7 years for an unspecified nephropathy, presented a brutal right facial paralysis, right hemicorpus dysesthesia and balance disorders. The magnetic resonance imaging (MRI) identified a lesion affecting the brain bridge, the right middle cerebellar peduncle and cerebellar hemisphere (Supplementary Fig. 1a, b). A stereotactic biopsy confirmed the diagnosis of diffuse large B-cell lymphoma with no other location assessed by positron emission tomography (PET) scan, establishing the diagnosis of PCNSL. EBV serology was negative. After multidisciplinary discussion, accordingly to the previous published data,4 the patient received 28 cycles of chemotherapy regimen R-MPV (Protocol described in Supplementary Methods). We used an adjusted the dose of MTX at 100mg/m2, based on the experience of one of the authors (unpublished).
Dialysis procedures was started 24h after MTX administration and conducted daily for four hours by predilution hemodiafiltration with high-flux dialyser, dialysate flow of 500mL/min, blood flow of 250mL/min, and high dialysate bicarbonate concentration (40mmol/L). Daily dialysis was discontinued when MTXc was <0.1μmol/L.
MTXc were measured before and after each dialysis session.
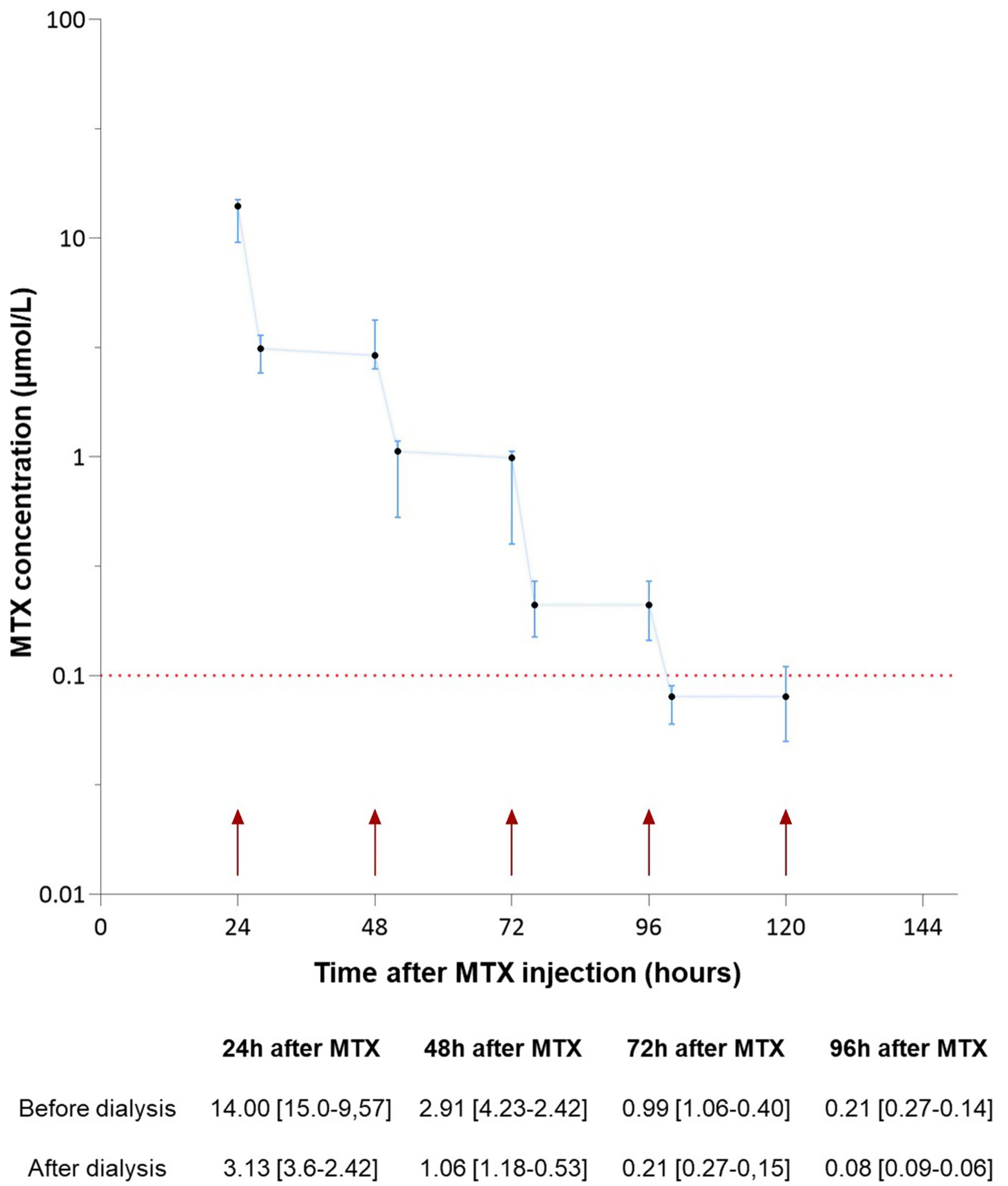
Median MTXc 24h after infusion was 14 [9.6–15.0]μmol/L (Fig. 1). No rebound phenomenon was observed. MTX was completely cleared on average at 5±0.6 days post-infusion.
Median [interquartile range] of plasma MTX concentration (μmol/L) following administration. Cycle 5 Days 15 was excluded. Small arrows represent dialysis sessions. The dashed line demarcates concentration at which folinic acid rescue was stopped. MTX: Methotrexate. MTXc: Methotrexate concentration.
Dose intensity was respected, and no course was delayed. Patient exhibited 2 episodes of undocumented sepsis with rapid favorable recovery, anemia requiring 2 red blood cells transfusion and mucositis.
Intermediary evaluation after 3 cycles with MRI assessment revealed a complete response.
The fifth cycle was marked by an unexplained MTX overdose at 180μmol/L 24h after administration, without clinical nor biological toxicity. The patient had an extended hemodialysis session of 16h in intensive care unit, achieving MTXc<0.5μmol/L and <0.1μmol/L after 1 more dialysis.
We thus decided to stop the chemotherapy treatment after 5 cycles, given the persistent complete response on MRI (Supplementary Fig. 1c, d) and brain PET-scan.
The patient is actually still in complete remission at 1 year.
We report here the feasibility of HD-MTX-like therapy in hemodialysis patients, and the first use of a dose divided by more than 10 from the standard dose. Herein, with an adjusted dose at 100mg/m2, with first hemodialysis session, our patient reached the expected MTXc at H24 and treatment was well tolerated with no significant toxicity and efficient with total recovery.
PCNSL is an aggressive type of lymphoma with a rapidly fatal course in the absence of treatment. HD-MTX-based chemoimmunotherapy have been shown to improve survival, even in elderly patients, and is a central part of the standard approach to treatment.2,4 Avoiding HD-MTX in hemodialysis patients leads to a loss of chance.
Although its important protein-binding and low distribution volume, 63% of MTX infused can be removed after a 6h high-flux hemodialysis session, four-fold faster than renal excretion.3
The strict monitoring of residual MTXc represents the major point for limiting its toxicity while keeping its effectiveness.
Five cases about HD-MTX use in hemodialysis patients have been reported (Table 1).3,5–8 The doses used were the same as in the general population (1–4g/m2). Some authors performed daily high-flux hemodialysis 24h after MTX infusion: cMTX were then very high (>20μmol/L) after the first dialysis session,6–8 above the threshold of toxicity.2 In any case, MTX was completely eliminated within 5–7 days after infusion.
Previously published cases of hemodialysis patients treated by HD-MTX.
| Study | Sex and age | Disease | Dose of MTX | Type and duration of HD | Time to HD initiation after infusion (h) | MTX plasma concentration 24h after the first infusion (μmol/l) | Patient evolution |
|---|---|---|---|---|---|---|---|
| Wall et al., 1996 | F13 y.o. | Osteosarcoma | 7.2g/m2 | High flux6h | 1 | 150 | Not specified |
| Mutsando et al., 2012 | F52 y.o. | Cerebral PTLD | 1g/m2 and escalating dose | High flux7h | 1 | 8 | Pancytopenia, gastrointestinal and hepatic toxicity, invasive infection with endocarditis.Death 4 month after 2 doses treatment from a CMV related perforated gastric ulcer. |
| Murashima et al., 2009 | M58 y.o. | PCNSL | C1 3g/m2C2 6g/m2then 4g/m2 | High flux6h | 24 | 100 | Not specified |
| Reshetenik et al., 2015 | M26 y.o. | Cerebral PTLD (second line) | 4g/m2 | High flux4h | 24 | 29 | Severe CMV and E.coli pneumonia requiring invasive ventilation and leading to stop chemotherapy |
| Yeung et al., 2019 | M41 y.o. | Cerebral PTLD | 1g/m2 and escalating dose | High flux8h | 24 | 22 | Clinical remission at 1 year after treatment |
CMV: cytomegalovirus, HD: hemodialysis, HD-MTX: high dose methotrexate, PCNSL: primary central nervous system non Hodgkin lymphoma, PTLD: post-transplantation lymphoproliferative disorder, y.o.: years old.
Importantly, we used a dose of 100mg/m2 every 2 weeks, and cMTX were close to those described in non-ESRD patients with PCNSL treated by HD-MTX.4,9 Our patient had moderate and reversible MTX-induced toxicity, and favorable outcome. ESRD patients may also have an increased efficacy of MTX at lower doses, due to a potentially increased blood-brain barrier permeability.10
We argue that ESRD is not an absolute pitfall to the use of HD-MTX for hematological malignancies. Experts should consider the use of adjusted dose at 100mg/m2 as a viable therapeutic modality in ESRD patients.
Statement of ethicsThe patient gave her informed consent for the publication of this case report.
Funding sourcesNone.
Conflict of interestNone.
We thank Dr. Dammar Bouchouareb, Julie Bruno, Mathilde Fedi & Denis Bontemps for participating in the patient's care.


![Median [interquartile range] of plasma MTX concentration (μmol/L) following administration. Cycle 5 Days 15 was excluded. Small arrows represent dialysis sessions. The dashed line demarcates concentration at which folinic acid rescue was stopped. MTX: Methotrexate. MTXc: Methotrexate concentration. Median [interquartile range] of plasma MTX concentration (μmol/L) following administration. Cycle 5 Days 15 was excluded. Small arrows represent dialysis sessions. The dashed line demarcates concentration at which folinic acid rescue was stopped. MTX: Methotrexate. MTXc: Methotrexate concentration.](https://static.elsevier.es/multimedia/20132514/0000004200000002/v4_202206160546/S2013251422000220/v4_202206160546/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w94GCRvdQBB6xyQjMrWMzrts=)




