Objetivo: Evaluar el impacto de una consulta específica de accesos vasculares (C-FAV) con seguimiento intensivo en la permeabilidad de las fístulas húmero-axilares. Pacientes y método: Estudio retrospectivo. Entre enero de 2005 y diciembre de 2009 se realizan 108 fístulas húmero-axilares. Desde junio de 2007 se establece una C-FAV. Se realiza eco-doppler preoperatorio y seguimiento posterior al mes de la intervención y, después, cada 3 meses. Resultados: Se analizan las permeabilidades de 57 fístulas húmero-axilares realizadas desde junio de 2007 hasta diciembre de 2009 (grupo C-FAV), comparándolas con 51 realizadas durante los 30 meses previos (grupo control). No se encontraron diferencias en la permeabilidad obtenida entre ambos grupos a 12 y 24 meses, con una permeabilidad secundaria a los 12 meses de 49% en el grupo C-FAV y 52% en el grupo control. El porcentaje de pacientes reintervenidos fue inferior en el grupo C-FAV (35%) que en el grupo control (67%), p = 0,002. La media de reintervenciones realizadas por paciente fue menor en C-FAV que en grupo control (0,49 vs. 1,18, p = 0,01). Los pacientes del grupo C-FAV presentaron un menor número de reintervenciones por obstrucción frente al grupo control (0,42 vs. 1,04, p = 0,01). Conclusiones: En nuestra experiencia, el seguimiento intensivo no ha mejorado la permeabilidad de las fístulas húmero-axilares, disminuyendo no obstante las reintervenciones por obstrucción. El seguimiento de estos accesos debe ser clínico basado en datos de hemodiálisis, quedando la valoración ecográfica para aquellos casos con sospecha de malfunción.
Aim: To evaluate the impact of a specific vascular access (arteriovenous fistula) unit (AVF-U) and intensive follow-up controls on the patency of humero-axillary fistulas (Hax-AVF). Patients and method: Retrospective study. Between January 2005 and December 2009, 108 Hax-AVF were implanted. From June 2007 an AVF-U was established. A preoperative Doppler ultrasonography analysis was performed and a follow-up control carried out a month after the intervention and subsequently every 3 months. Results: An analysis was made of the patency of 57 Hax-AVF performed between June 2007 and December 2009 (AVF-U Group), in comparison to 51 interventions performed during the previous 30 months (Control Group). No differences in the patency achieved were found at 12 or 24 months, with a secondary permeability at 12 months of 49% in the AVF-U Group and 52% in the Control Group. The percentage of patients needing to be reoperated was lower in the AVF-U Group (35%) than in the Control Group (67%) (P=.02). The mean number of re-operations per patient was lower in the AVF-U Group than in the Control Group (0.49 vs 1.18; P=.01). The patients of the AVF-U Group underwent fewer reoperations for obstruction as compared to the Control Group (0.42 vs 1.04; P=.01). Conclusions: In our experience, the intensive follow-up controls did not improve the patency of the Hax-AVF, although reoperations due to obstruction did diminish. The follow-up of these fistulas should be clinically based on haemodialysis data, leaving ultrasound evaluation for those cases where AVF failure is suspected.
INTRODUCTION
The number of patients on haemodialysis in Spain has increased in recent years. An estimated 45 000 patients are on renal replacement therapy in Spain (1000 patients per 1000 000 inhabitants).1 In addition, this population is aging and show an increasing prevalence of diabetes, accompanied by an increased proportion of prosthetic grafts or arteriovenous fistulas (AVF).2
Prosthetic AVF are associated with increased morbidity, as well as lower primary and secondary patency as compared to native AVF, and require reoperations more often.3 As such, these vascular accesses continue to produce substantial rates of hospitalisations and morbidity in patients on haemodialysis, and they are a constant source of headache for nephrologists and the nursing staff charged with daily patient management in haemodialysis units.
Primary and secondary patency of prosthetic vascular accesses continue to be lower than desired so in recent years growing interest has been devoted to preoperative mapping strategies and follow-up regimens, both in haemodialysis sessions by nephrologists and in visits for high resolution imaging of vascular accesses.4
Preoperative evaluation with Doppler ultrasound before creating a new vascular access is currently recommended by KDOQI and European guidelines. Several studies in the medical literature have correlated preoperative mapping by Doppler ultrasound with a significant increase in primary patency of the fistula.5 However, more recent studies have placed doubts in the usefulness of Doppler ultrasound as a strategy for improving patency in prosthetic arteriovenous grafts.6
Contrasting results have been reported in studies of follow-up protocols for vascular accesses. Observational studies have suggested that Doppler ultrasound is useful for improving vascular access results, whereas randomised studies have come to the opposite conclusion.6 In this sense, intensive clinical follow-up by the nursing staff in haemodialysis units plays a key role. We should also highlight the role of the nephrologist as a driving force between the detection of the problem by the nurse and resolution of the issue by the vascular surgeon responsible for the vascular access.
The aim of our study was to evaluate the impact of a specific unit for vascular accesses and intensive follow-up on the patency of humero-axillary AVF.
PATIENTS AND METHOD
Between January 2005 and December 2009, 108 humero-axillary AVF were performed at our hospital. In June 2007, a specific unit for vascular accesses was established, in which preoperative Doppler ultrasound tests were performed, following the recommendations of the KDOQI guidelines,7 with follow-up sessions one month after the procedure and every three months afterwards. These intervals were shortened if the attending nephrologist deemed it necessary.
The 108 patients were divided into 2 groups: a control group, which consisted of 51 fistulas that were created before June 2007, when the vascular access unit was established, and the vascular access unit (AVF-U) group, which included 57 fistulas performed between June 2007 and December 2009. Ours was a retrospective study, comparing two different time periods separated by this newly established protocol. We also recorded demographic variables such as age, sex, and vein and artery diameters using preoperative ultrasound mapping. The clinical variables evaluated were diabetes mellitus (DM), arterial hypertension (AHT), and dyslipidaemia (DL).
The preoperative assessment in the control group consisted of a physical examination and venography in select cases. The AVF-U group was assessed using preoperative Doppler ultrasound, measuring humeral artery and axillary vein diameters. Venography was indicated in cases where indirect signs of central venous occlusion or stenosis were observed.
Follow-up during haemodialysis treatment was established in both groups. Criteria for AVF failure included dynamic venous pressure >200mm Hg or flows <350ml/min. An absence of pulse or murmurs upon auscultation was considered indicative of an obstructed access, which was confirmed using Doppler ultrasound in the AVF-U group.
In the AVF-U group, we also performed follow-up sessions with Doppler ultrasound one month after the intervention and every three months afterwards, except when the nephrologist suspected of AVF failure and indicated further evaluations. At each follow-up visit, we measured peak systolic velocity in the proximal artery, arterial anastomosis, pathway, venous anastomosis and distal vein, as well as the flow rate at the middle third of the pathway. We defined significant stenosis as an increase in peak systolic velocity >2x in the AVF pathway or >4x in the anastomoses, along with an image indicative of a stenosis. In addition, a flow rate <400ml/min was considered pathological.
For the statistical analysis, we used SPSS software version 15.0 for Windows, and performed chi-square, Student’s t-tests, and Kaplan-Meier survival curves. Kaplan-Meier curves were compared using Breslow tests. We established the significance level at P<.05.
RESULTS
We performed a total of 108 consecutive humero-axillary AVF between January 2005 and December 2009. Of them, 51 were created before the specific unit for vascular accesses (control group) was established (June 2007), and 57 humero-axillary AVF were performed after this date (AVF-U group).
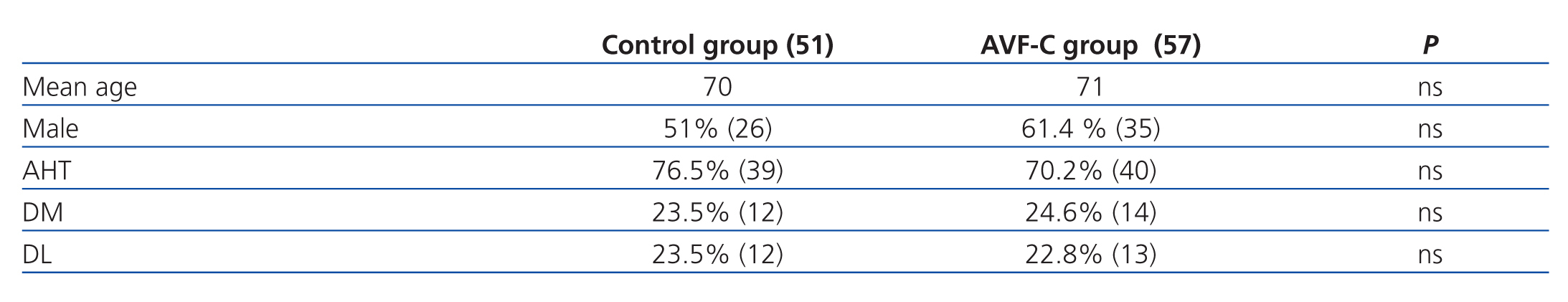
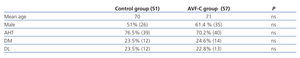
In the control group, 51% of patients (26) were male, and the mean age was 70 years; in the AVF-U group, 61.4% (35) were male, and the mean age was 71 years. We determined AHT, DM, and DL, and found no significant differences between the two groups in any of the three measures, as shown in Table 1.
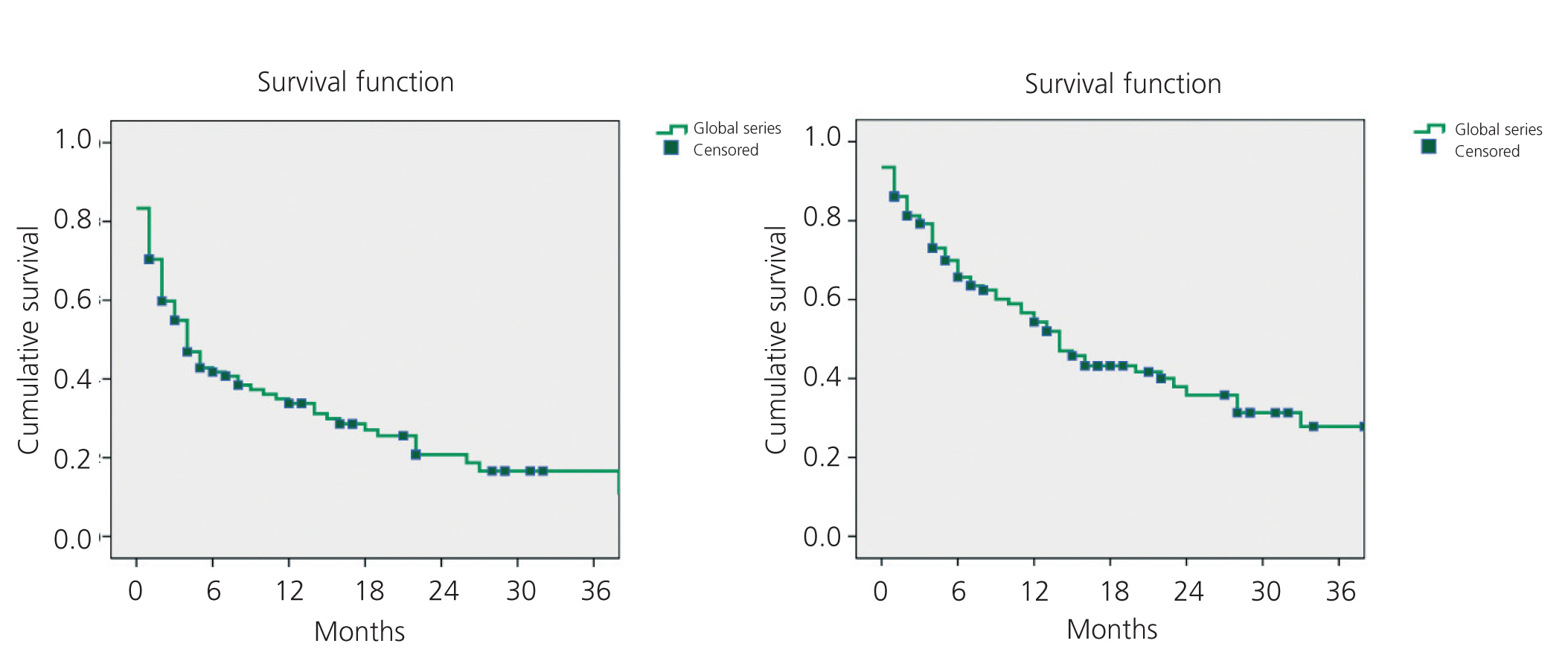
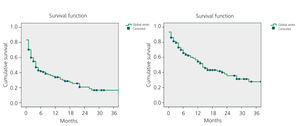
The mean primary patency for all 108 AVF was 34% after 12 months and 20% after 24 months. Secondary patency was 54% after 12 months and 36% after 24 months, as shown in Figure 1.
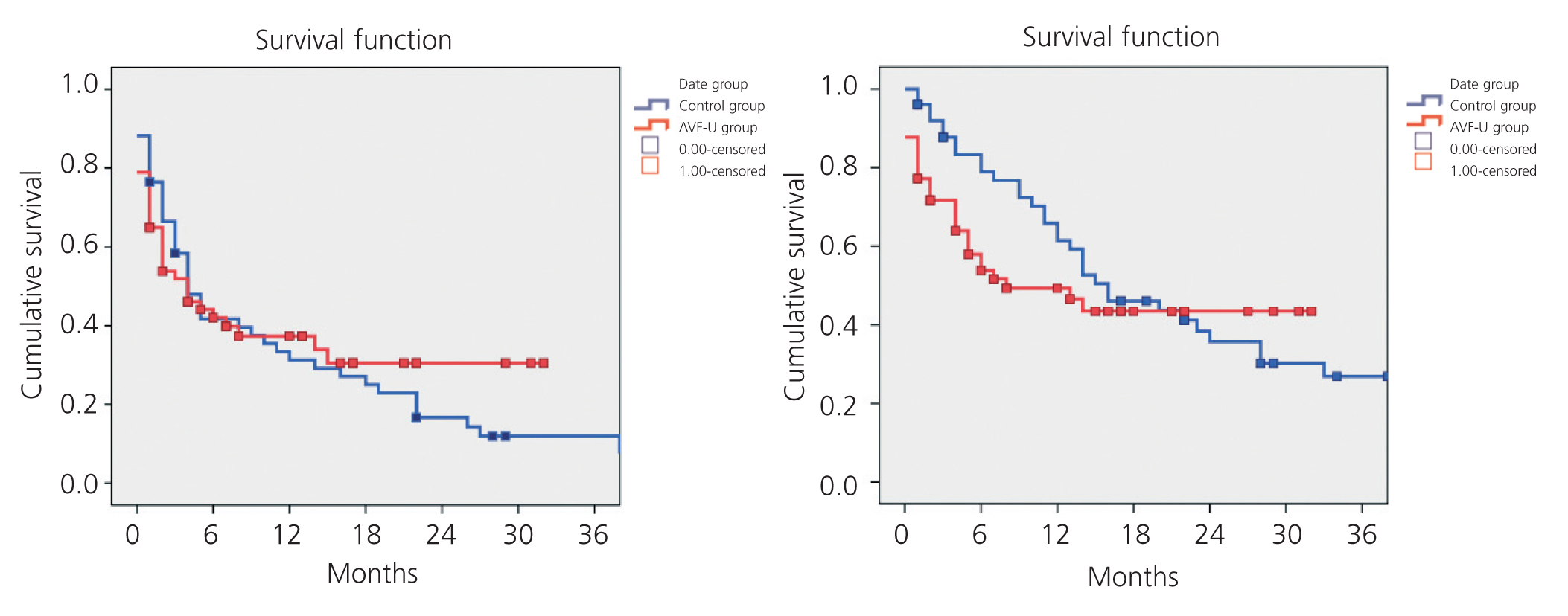
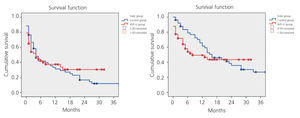
While primary patency was higher in the AVF-U group, the difference as compared to the control group was not statistically significant. Nor did we observe significant differences in secondary patency between the two groups, as shown in Figure 2.
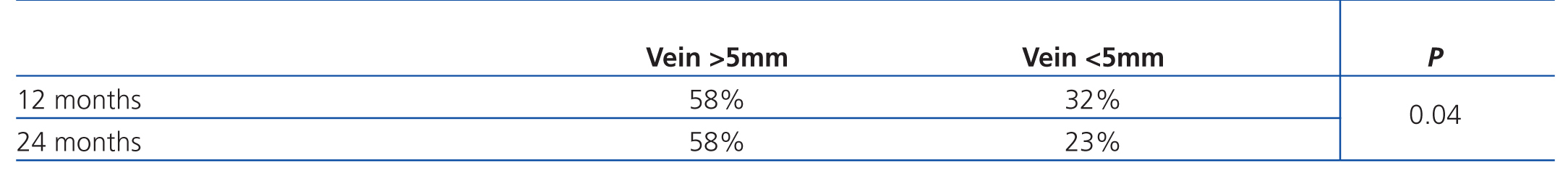
We analysed possible prognostic factors and observed a significantly lower primary patency value in humero-axillary fistulas with a vein diameter <5mm (Table 2). In the analysis by sex, we observed a lower primary patency in women, which was associated with a lower venous diameter, but no differences were statistically significant.
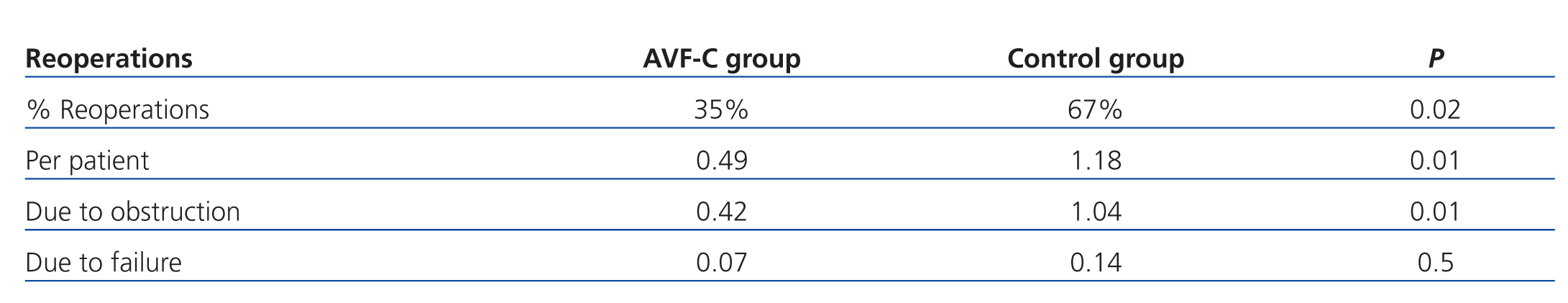
The percentage of patients requiring reoperations in the control group was twice that of the AVF-U group (P=.02). The mean number of reoperations per patient in the control group was 1.18 as compared to 0.49 in the AVF-U group (P=.01).
We analysed the causes of reoperations and observed that reoperations due to obstruction were significantly fewer in the AVF-U group, with a mean 0.42 reoperations per patient vs 1.04 in the control group (P=.01). However, when analysing the reoperations due to AVF failure, there were no significant differences between groups (control group: 0.14; AVF-U group: 0.07; P=.5). The reoperations performed by group are summarised in Table 3.
DISCUSSION
Since Brescia-Cimino described radio-cephalic AVF in 1966, autologous vascular accesses have become the method of choice due to the positive results obtained in primary and assisted patency and the low rate of complications.8
Several studies have demonstrated an increase in the life expectancy of patients on haemodialysis, an increase in the prevalence of DM, higher rates of long-term dialysis patients, and increased use of high-efficiency dialysis. This change in demographics of the population on dialysis treatment has led to an increasing use of prosthetic AVF, strategies for preoperative mapping, and Doppler ultrasound follow-up regimens in order to improve the long-term patency of vascular accesses.3
The usefulness of vessel mapping by Doppler ultrasound in preoperative studies for AVF has been recognised for many years. Doppler ultrasound yields very useful information regarding the diameter, level of calcification, and presence of haemodynamically significant lesions in radial and humeral arteries. These factors have been employed as predictive factors for the maturation period of an AVF.9 They also provide information regarding patency, distensibility, and diameter of the veins being analysed, which is of great importance when selecting the optimal vein for performing the AVF, according to a study by Van der Linden et al10 from 2007. Silva et al11 highlighted the importance of preoperative mapping by Doppler ultrasound, concluding that preoperative Doppler ultrasound reduces the rate of early AVF thrombosis and increases the percentage of autologous AVF.
In contrast, Nursal et al12 concluded that a physical examination is sufficient for determining which vascular access point is best, and if the patient’s anatomy is favourable, a Doppler ultrasound analysis is not necessary for adequate planning of an AVF.
In our study, Doppler ultrasound preoperative mapping did not increase significantly the primary or secondary patency of humero-axillary AVF, which is in accordance with a Canadian meta-analysis6 published in 2008, in which no evidence was found to demonstrate a benefit of preoperative mapping with Doppler ultrasound in prosthetic AVF.
Silva et al performed a study in 1998 in which they analysed patency values by vein diameter as measured by preoperative Doppler ultrasound, and established a minimum venous diameter of 4mm for prosthetic AVF. In our study, we observed that primary patency was significantly lower in humero-axillary AVF with an axillary venous diameter <5mm.3,11
As regards the follow-up of prosthetic AVF, contrasting results can be found. Several observational studies highlighted the importance of Doppler ultrasound follow-up of AVF for haemodialysis, describing an increased patency and decreased need for reoperations.13 Shemesh14 in 2004 published a study in which a secondary patency of over 80% 3 years after the establishment of the AVF, patients having undergone a preoperative mapping analysis and a strict follow-up of the prosthetic graft by Doppler ultrasound. However, a 2008 meta-analysis involving 12 randomised studies and a total of 1570 patients analysed the impact of a follow-up regimen with imaging tests of vascular accesses along with early intervention to re-establish patency. Of these studies, 9 did not found a significant decrease in the risk of thrombosis of the vascular access with the follow-up regimen, although such a result was observed in the other 3 studies.15 According to Akoh et al,3 no reasons are found for prosthetic AVF thrombosis in 20% of cases, which could explain the difficulty in monitoring these vascular accesses.
In our study, we observed higher primary and secondary patency of humero-axillary AVF in the AVF-U group, although this difference was not statistically significant. Furthermore, we did observe a decrease in both the number of reoperations per patient and the number or reoperations due to obstructions in the AVF-U group. This could be due to a better identification of vascular accesses with a poor prognosis, and early reoperations in failing AVF. We must keep in mind that, since the specific vascular access unit was established, the procedures performed for AVF have been carried out mainly by the vascular surgery department, instead of the interventional radiology one. Vascular surgeons perform the vast majority of vascular access repairs, providing the best solution for each case (conventional or endovascular surgery), which may also have influenced the results, since the surgeon is capable of identifying with a greater level of accuracy those accesses in which a repair is contraindicated due to a poor prognosis. In our case, the interventional radiologist plays an important supporting role in the treatment of obstructive/stenosing lesions of the central veins, where radiological equipment available is particularly necessary.
CONCLUSION
The establishment of a specific unit for vascular accesses with intensive ultrasound follow-up did not improve the patency of humero-axillary AVF. Preoperative mapping by Doppler ultrasound allowed us to identify fistulas with a greater risk of obstruction, and an axillary vein diameter <5mm is a factor for a worse prognosis of the patency of humero-axillary AVF. Although intensive follow-up decreased the number of reoperations needed in these accesses due to obstruction, and Doppler ultrasound can be a very useful tool in monitoring these fistulas, the follow-up of these vascular accesses should take place in a clinical context, based on data from haemodialysis sessions. Ultrasound should only be used in cases of suspected AVF failure. In these cases, the nephrologist plays a key role, and communication between the nephrologist and the vascular surgery team charged with maintaining the vascular access is also of utmost relevance so as to achieve the final objective of this procedure, which is to extend the life span of vascular accesses in haemodialysis.
Conflicts of interest
The authors affirm that they have no conflicts of interest related to the content of this article.
Figure 1. Kaplan-Meier. Overall study results. Primary and secondary patency
Figure 2. Kaplan-Meier. Primary and secondary patency. Control vs AVF-U group
Table 1. Demographic characteristics and associated cardiovascular risk factors
Table 2. Primary patency according to venous diameter
Table 3. Reoperation